Introduction
Cellular immunotherapy, which harnesses the patient’s own immune system to recognize and eliminate cancer cells, has led to a major breakthrough in cancer treatment. At the forefront of this advancement is chimeric antigen receptor T-cell therapy (CAR T-cell therapy), a cutting-edge approach that involves genetically engineering a patient’s T-cells to target specific cancer antigens. This approach has shown remarkable success, demonstrating high response rates and lasting remissions in patients with otherwise limited treatment options, with seven CAR T-cell therapies having been approved by the U.S. Food and Drug Administration (FDA) to date. However, there are still challenges associated with CAR T-cell therapy, such as limited efficacy against solid tumors, potentially severe and life-threatening side effects, high production costs, lengthy manufacturing process, and limited scalability. These challenges have spurred interest in exploring alternative immune effector cells for CAR-based therapy, with natural killer (NK) cells emerging as a promising candidate.
Advantages of CAR-NK cell therapy
NK cells are a type of innate immune cell that plays an integral role in the body’s first line of defense against infections and transformed cells. Unlike T-cells that require antigen presentation by major histocompatibility complex (MHC) to become activated, NK cells can recognize and kill cancer cells without prior sensitization or antigen recognition. Because NK cells are not limited by MHC restriction, they can target a broader range of cancers, and they also have a lower likelihood of causing severe side effects, such as cytokine release syndrome and graft versus host disease (GvHD). Additionally, NK cells possess an inherent ability to target and kill cancer cells through a variety of mechanisms, including direct cytotoxicity, antibody-dependent cellular cytotoxicity, and the release of cytotoxic granules. These attributes have made NK cells an attractive candidate for CAR-based therapy.
How CAR-NK cell therapy works
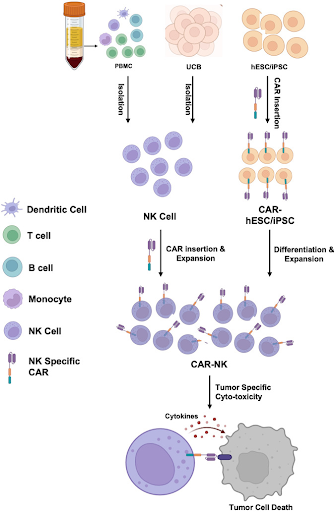
The process of creating CAR-NK cells involves isolating NK cells from the patient’s own blood or from the blood of a healthy donor. In addition to peripheral blood, other sources of NK cells include umbilical cord blood (UCB), NK cell lines, as well as stem cells such as human embryonic stem cell (hESC) and induced pluripotent stem cell (iPSC). Each source of NK cells has its own advantages and limitations, with some allowing the development of “off-the-shelf” products that are readily available, thus reducing time and costs associated with the individualized manufacturing of autologous therapies. After the NK cells are genetically engineered in the laboratory to express a CAR targeting specific cancer antigen, the CAR-NK cells are infused into the patient, where they seek out and destroy cancer cells that carry those specific antigens.
Challenges of CAR-NK cell therapy
Despite its vast potential, CAR-NK cell therapy is still in its early stages and faces several challenges. One major hurdle is its limited persistence, as NK cells have a shorter lifespan and weaker memory compared to T-cells, which can limit the long-term efficacy of CAR-NK cell therapy. Additionally, the dense tumor stroma, immunosuppressive factors, and hypoxic conditions in solid tumors can all hinder the effectiveness of immunotherapies, including CAR-NK cell therapy. Similar to CAR T-cell therapy, antigen escape is also a challenge in CAR-NK cell therapy. Tumors can downregulate or lose the expression of the target antigen, rendering CAR-NK cells ineffective. To overcome these challenges, researchers are actively exploring different strategies to enhance the durability and efficacy of CAR-NK cells, such as engineering them to secrete cytokines like interleukin-15 (IL-15) to promote survival and persistence in the body, combining CAR-NK cells with other treatments like immune checkpoint inhibitors to improve efficacy, and developing CAR-NK cells with dual or even multiple antigen targeting capabilities.
Future of CAR-NK cell therapy
While no CAR-NK cell therapy has been approved by the U.S. FDA yet, significant progress is being made with promising results in early-phase clinical trials, particularly for certain hematological malignancies such as lymphomas. These trials have demonstrated a good safety profile and efficacy, sometimes even in patients who did not respond to CAR T-cell therapy. Their potential to be developed as an “off-the-shelf” therapy is particularly attractive. NK cells from healthy donors, cell lines, or stem cells can be engineered, expanded and stored in large cell banks. This approach offers several advantages like reduced treatment waiting times for patients and potential cost savings through improved manufacturing scalability, making CAR-NK cell therapy more accessible. As research and clinical trials continue to advance, CAR-NK cell therapy is poised to play a pivotal role in the next generation of cancer therapies, offering a safer, more versatile, and potentially more effective alternative to existing therapies.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.