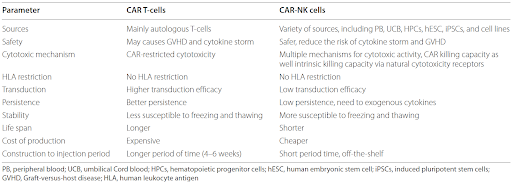
A Comparative Overview of CAR T-Cell and CAR-NK Cell Therapies
In recent years, chimeric antigen receptor (CAR) cell therapies have transformed cancer treatment by offering a promising option for patients who previously experienced limited success with conventional therapies. CAR T-cell therapy, which harnesses the power of genetically modified T cells to target and eliminate cancer cells with precision, has led to unprecedented long-term remissions in patients with relapse or refractory cancers. Given its remarkable success, researchers have continued to explore other immune cells for CAR-based therapy, such as natural killer (NK) cells. Although both CAR T-cell and CAR-NK cell therapies involve genetically engineered receptors that enable them to specifically target cancer cells by recognizing tumor-associated antigens, they differ in several important ways.
Source:
CAR T-cells are typically derived from the patient’s own peripheral blood, requiring individualized, time-consuming manufacturing. Although there are on-going efforts to develop donor-derived CAR T-cells, these T-cells require further genetic engineering to prevent rejection from patient’s immune system and graft-versus-host disease (GvHD). On the other hand, CAR-NK cells can be sourced from a healthy donor’s peripheral blood, umbilical cord blood, stem cells, or NK cell lines, enabling their use as an allogeneic therapy.
Mechanism of action:
T-cells are part of the adaptive immune system and rely on the major histocompatibility complex (MHC) molecules to present antigens found on the surface of cancer cells. Upon recognition, T-cells become activated and release cytotoxic molecules like perforin or granzyme, to induce apoptosis in the targeted cancer cell. In contrast, NK cells are part of the innate immune system and do not depend on MHC molecules for antigen presentation. They can directly detect and kill cancer cells through several mechanisms, including direct cytotoxic activity, antibody-dependent cellular cytotoxicity, and the secretion of cytotoxic granules. These processes can be triggered by the presence of stress signals or abnormal markers on the tumor surface, allowing NK cells to act faster without prior sensitization.
Side effects:
One of the key challenges with CAR T-cell therapy is the potential severe side effects, which can lead to death. The most notable of these is cytokine release syndrome (CRS), which occurs when the CAR T-cells become overly activated and release large amounts of pro-inflammatory cytokines into the bloodstream. CRS can lead to inflammation, fever, organ damage, and even death. Immune effector cell-associated neurotoxicity syndrome (ICANS) is another serious side effect that can cause cognitive dysfunction and motor impairments. In severe cases, ICANS can progress to encephalopathy, coma, cerebral edema, or death. Since NK cells do not undergo the same extensive activation process as T-cells and produce fewer pro-inflammatory cytokines, they offer a safer profile than CAR T-cells, with a lower likelihood of severe side effects like CRS and ICANS.
Persistence:
One of the hallmarks of CAR T-cell therapy is their long-term persistence in the body. After infusion, CAR T-cells can remain active and continue to proliferate for weeks or even months, providing a durable response, a key factor in the therapy’s clinical success. In contrast, CAR-NK cells have a shorter persistence in the body compared to CAR T-cells. The shorter lifespan and limited proliferative capacity of NK cells can limit their effectiveness in cases where sustained immune responses are needed, although this can also be advantageous in reducing the risks of severe side effects associated with prolonged immune activation, such as CRS and ICANS.
Manufacturing:
Since CAR T-cells are typically developed from the patient’s own T-cells, it requires complex and lengthy manufacturing. This process can take several weeks, making CAR T-cell therapy less accessible for patients in urgent need of treatment. Additionally, this patient-specific manufacturing is also costly, which further limits accessibility for a broader range of patients. CAR-NK cells, on the other hand, have the potential to be produced “off-the-shelf”, by sourcing the NK cells from healthy donors, stem cells, or NK cell lines. Because they can be manufactured in large quantities and distributed to patients without requiring a perfect donor match, this could lead to significant cost reductions and faster treatment availability, making the therapy more accessible.
Current status:
The first CAR T-cell therapy approved by the U.S. Food and Drug Administration (FDA) was Kymriah™ (tisagenlecleucel) on August 30th, 2017, for the treatment of acute lymphoblastic leukemia. Since then, six more CAR T-cell therapies have been approved by the U.S. FDA for various indications: Yescarta™ (axicabtagene ciloleucel) for large B-cell lymphoma and follicular lymphoma in 2017; Tecartus™ (brexucabtagene autoleucel) for mantle cell lymphoma and acute lymphoblastic leukemia in 2020; Breyanzi™ (lisocabtagene maraleucel) for large B-cell lymphoma, lymphocytic leukemia, follicular lymphoma, and mantle cell lymphoma in 2021; Abecma™ (idecabtagene vicleucel) for multiple myeloma in 2021; Carvykti™ (ciltacabtagene autoleucel) for multiple myeloma in 2022; and Aucatzyl™ (obecabtagene autoleucel) for acute lymphoblastic leukemia in 2024. On the other hand, CAR-NK cell therapy is still largely in the experimental phase, with early-phase clinical trials demonstrating promising results. Published studies have reported favorable clinical responses among patients with advanced hematological malignancies (acute myeloid leukemia, acute lymphoblastic leukemia, and lymphoma) and solid tumors (glioblastoma, pancreatic cancer, and lung cancer), with very low rates of adverse side effects such as CRS, ICANS, or GvHD.
In summary, both CAR T-cell and CAR-NK cell therapies represent cutting-edge advancements in cancer immunotherapy, each offering distinct advantages and challenges. CAR T-cell therapy has demonstrated impressive success in treating various hematological malignancies, but its potential severe side effects, limited efficacy in solid tumors, as well as lengthy and costly manufacturing process remain areas of concern. On the other hand, CAR-NK cell therapy has emerged as a promising alternative with a better safety profile, greater versatility, and the potential to be developed as an “off-the-shelf” therapy. However, CAR-NK cells are still in the early stages of clinical development and face challenges related to durability and long-term effectiveness. As research progresses, the future of cancer immunotherapy may lie in combining the strengths of both therapies, leveraging the unique properties of T-cells and NK cells to create more effective, safer, and personalized treatment options for patients with various cancer types.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.