Introduction
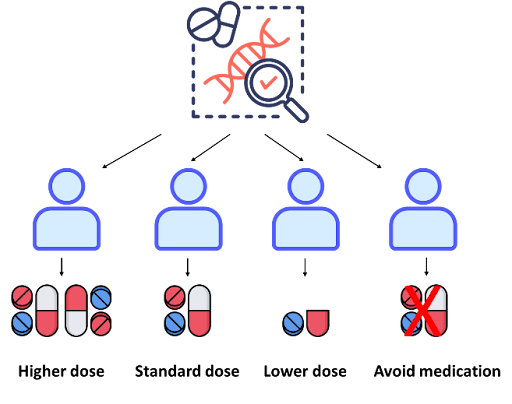
One area of focus in cancer pharmacogenomics is looking at how germline variations in individuals can affect their response to cancer therapies. Germline variants are present in all the body’s cells, including the germ cells (egg or sperm), and can therefore be inherited by offspring. Single nucleotide variants (SNVs), which involves substitution of a single nucleotide for another, are the most common type of genetic variation in humans. If an SNV is present in at least 1% of the population, it is then known as a single nucleotide polymorphism (SNP). SNPs are abundant in the human genome, occurring almost once in every 500 to 1,000 bases on average. Studies have demonstrated that germline SNPs in certain genes (known as pharmacogenes) can influence the metabolism of cancer drugs, impacting their efficacy and/or toxicity. As a result, pre-emptive genetic testing for these pharmacogenes has been included into clinical guidelines such as the U.S. Food and Drug Administration (FDA) and Clinical Pharmacogenetics Implementation Consortium (CPIC). Here are some examples:
TPMT/NUDT15 and thiopurines
TPMT (thiopurine S-methyltransferase) and NUDT15 (nudix hydrolase 15) are genes involved in metabolizing thiopurines, such as mercaptopurine and azathioprine, which are commonly used in treatment of hematological malignancies. Patients with certain germline variants in these genes may not be able to metabolize these drugs effectively, leading to higher levels of thiopurines in the body and elevating the risk of severe side effects such as myelosuppression.
DPYD and fluoropyrimidines
Fluoropyrimidines such as 5-fluorouracil (5-FU) are commonly used in the treatment of various cancers. The dihydropyrimidine dehydrogenase (DPD) enzyme, encoded by the DPYD gene, is involved in the metabolism of 5-FU and is responsible for eliminating more than 80% of administered 5-FU. Patients with certain variants in DPYD gene can have reduced or absent DPD enzyme activity, leading to increased levels of 5-FU in the body and putting them at higher risk of toxicity.
UGT1A1 and irinotecan
UGT1A1 (UDP-glucuronosyltransferase 1A1) is a gene that encodes an enzyme involved in the metabolism of irinotecan, a chemotherapy drug used to treat various solid tumors. UGT1A1 is responsible for detoxifying and eliminating irinotecan and its active metabolite, SN-38, from the body. Genetic variants such as UGT1A1*28 polymorphism reduces the metabolism of SN-38, increasing the risk of irinotecan-induced toxicities, including severe neutropenia and diarrhea in patients treated with irinotecan.
CYP2D6 and tamoxifen
CYP2D6 (cytochrome P450 2D6) is a gene that is highly polymorphic and involved in metabolism of up to 25% of the drugs that are commonly used, including cancer drugs like tamoxifen. It is estimated that around 5-10% of the population have two non-functional alleles (poor metabolizers) and up to 30% of the population, depending on ethnicities, have duplications of functional alleles (ultra-rapid metabolizers). CYP2D6 enzyme metabolizes tamoxifen to its active form, endoxifen, to block estrogen receptors on breast cancer cells. Poor metabolizers may have reduced conversion of tamoxifen to endoxifen, potentially leading to lower drug effectiveness. On the other hand, ultra-rapid metabolizers may convert tamoxifen to endoxifen too rapidly, which may increase the risk of adverse effects. Although genotype-based dosing has demonstrated real-world evidence on its value, this is still not routinely implemented in the clinic, with most drugs being prescribed using a “one-dose-fits-all” approach. By integrating pre-emptive genetic testing of pharmacogenes into cancer treatment plans, drug dosage can be tailored for each patient to maximize drug efficacy and minimize drug-related toxicity, thus bringing us closer to truly personalized medicine.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.