Introduction
Somatic variants are genetic mutations that arise in somatic cells, which are any cells in the body except for germ cells (egg and sperm cells). These mutations are not inherited but occur spontaneously as a result of errors in DNA repair mechanisms or random mutations induced by environmental factors (e.g., UV radiation and carcinogenic chemicals). Sporadic cancers, which are driven by somatic mutations, account for around 75-80% of all cancer cases. Somatic mutations can be categorized into driver mutations and passenger mutations. Driver mutations play a direct role in cancer development and progression by conferring selective advantages to cells in their microenvironment, enabling them to grow and divide uncontrollably, eventually becoming cancerous. On the other hand, passenger mutations do not provide any fitness advantage to the cells and therefore do not contribute directly to the development and progression of the disease. Hence, identifying driver mutations is pivotal in cancer management to enable more accurate diagnosis, prognosis, and treatment strategies, leading to improved patient outcomes.
Detecting cancer-related variants with NGS
In recent years, assays for single-target detection are increasingly being replaced by next-generation sequencing (NGS). NGS is a high-throughput platform capable of analyzing many targets and identifying multiple types of mutations simultaneously in a single assay. Therefore, it is not surprising that NGS can identify a large number of variants from tumor tissue. This can be attributable to the complexity of carcinogenesis, including the multistep process of genetic mutations and tumor heterogeneity. However, it is estimated that only a handful of variants identified are driver mutations with clinical significance, while the majority are passenger mutations. Variant classification is a systematic process by which clinically significant variants are winnowed out from the tumor’s molecular profile to guide the clinical management of cancer patients. This information can offer diagnostic and prognostic insights, guide therapy selection or identify clinical trials with investigational agents based on mutation detected.
AMP/ASCO/CAP recommendations on somatic variant classification
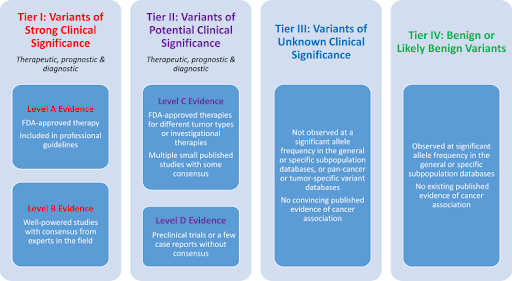
Given the crucial nature of variant classification and importance of standardizing the interpretation and reporting of variants detected during cancer sequencing, a multidisciplinary working group was convened by the Association for Molecular Pathology (AMP), with liaison representatives from the American College of Medical Genetics and Genomics (ACMG), American Society of Clinical Oncology (ASCO), and College of American Pathologists (CAP) to assess the current status of NGS-based cancer testing and establish standardized consensus classification, annotation, interpretation, and reporting conventions for somatic sequence variants. Based on the results of professional surveys, literature review, and the working group’s subject matter expert consensus, a four-tiered evidence-based system to categorize somatic variants based on their clinical impact in cancer diagnosis, prognosis and/or therapeutics was recommended: Tier I, variants of strong clinical significance; Tier II, variants of potential clinical significance; Tier III, variants of unknown clinical significance; and Tier IV, variants deemed benign or likely benign. The evidence used for the somatic variant classification is derived from a broad number of resources, such as U.S. Food and Drug Administration (FDA)-designated biomarkers, professional guidelines, clinical trials, function of the variants, population databases, variant databases, predictive algorithms, and published literature.
Update to the 2017 AMP/ASCO/CAP recommendations on somatic variant classification
Since it was published in 2017, the recommendations have been widely adopted by cancer diagnostics laboratories worldwide. To maintain the continued relevance and clinical utility of the tiered categorization system, a multidisciplinary working group was convened by the AMP in 2022 to update the recommendations by incorporating real-world experience and addressing community feedback. The draft recommendations are currently available on AMP website for public review and comment until the end of February 2025. After this period, the final recommendations will be published as an update to the 2017 standards and guidelines recommendations, based on the comments received and evidence gathered from the refreshed literature search. With tumor molecular profiling becoming standard of care, this update aims to codify somatic variant interpretation and reporting, ultimately improving clinical outcomes and patient care.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.