Transforming Cancer Care Through Innovation and Immunotherapy
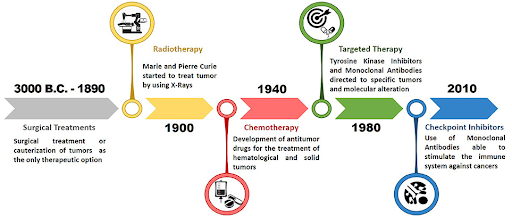
Over the last century, cancer treatment has evolved at a remarkable pace. Beginning with invasive surgical procedures, the field has advanced from the general approaches of radiotherapy and chemotherapy to the precision of targeted therapy, and now, to harnessing the body’s own immune system through immunotherapy. The shift from the crude methods of early days to today’s cutting-edge therapies has been transformational, and has significantly improved prognosis and the quality of life for cancer patients worldwide.
Surgery
Surgical intervention is the oldest treatment for cancer, with its origins dating back to ancient civilizations. The first recorded use of surgical procedures can be traced back to the ancient Egyptians, where a papyrus described a case of breast cancer. It suggested a treatment involving cauterization followed by bandaging with therapeutic herbs for relief. A 2024 study revealed cut marks on a 4,000 years old Egyptian skull with lesions believed to be from malignant tumors. Although it was not known if these cuts were part of a treatment attempt or an autopsy, this finding indicates that surgery was practiced by the ancient Egyptians. Early surgeries were often crude, and without anesthesia and good understanding of infection control, they often did more harm than good. The discovery of anesthesia and aseptic techniques in the 19th century marked a turning point, significantly improving surgery outcomes and allowing surgeons to perform more extensive procedures. One of the most notable examples was the radical mastectomy, pioneered by William Halsted in the late 1880s. This aggressive procedure, which involved the removal of the breasts, chest muscle, and lymph nodes, became the standard of care for nearly a century. However, it became evident over time that surgery alone was not sufficient to treat cancer, especially in cases where the disease has spread beyond the initial tumor site.
Radiotherapy
Radiotherapy, or radiation therapy, uses high-energy radiation to kill cancer cells by damaging their DNA and thus preventing their growth. The use of radiotherapy dates back to the late 19th century, with the discovery of X-rays by Wilhelm Roentgen in 1895. The subsequent discovery of radium, a highly radioactive element, by Marie and Pierre Curie in 1898, paved the way for its use in treating cancer. Initially studied for diagnostic purposes, it was soon realized that the radiation from radium could destroy cancer cells, forming the foundation of modern radiotherapy. However, this approach only began to gain traction in 1920 when Claudius Regaud demonstrated that fractionating the radiation doses could effectively treat cancer while minimizing side effects on healthy tissue. From that point on, radiotherapy became a cornerstone of cancer treatment, offering an alternative to surgery. It is especially effective for treating localized tumors, and recent advances in imaging technologies and radiation delivery techniques have significantly enhanced the precision and safety of the therapy.
Chemotherapy
The development of chemotherapy can be traced back to the use of mustard gas during World War II. After observing that soldiers exposed to mustard gas experienced a significant drop in their white blood counts, it was hypothesized that mustard gas can be used to target fast-growing cancer cells. In a groundbreaking 1942 study conducted by Alfred Gilman and Louis Goodman, nitrogen mustard, a derivative of mustard gas, was administered to a patient with non-Hodgkin’s lymphoma, which led to the temporary but dramatic shrinking of the tumor. This clinical trial marked the first successful use of a chemical agent in treating cancer, a pivotal milestone in cancer therapy. The full results, published in 1946, sparked a new era of systemic cancer treatments and accelerated the development drugs designed to target and destroy rapidly dividing cells, a hallmark of cancer. Unfortunately, these chemotherapeutic drugs are not cancer-specific, and as a result, can lead to side effects such as hair loss, nausea, fatigue, and a weakened immune system. Despite these drawbacks, chemotherapy has saved countless lives and remains a vital tool in cancer care today.
Targeted therapy
A growing understanding of molecular and cellular biology in the late 20th century enabled researchers to understand the molecular mechanisms underlying carcinogenesis. In particular, the discovery of oncogenes and tumor suppressor genes led to the idea that therapies could be developed to target specific molecules or mutations driving cancer progression. This approach would offer a more precise alternative to chemotherapy, which indiscriminately targets all rapidly dividing cells. Tamoxifen, which binds to estrogen receptors to block the growth-promoting effects of estrogen on breast cancer cells, was approved by the U.S. Food and Drug Administration (FDA) in 1977 for the treatment of estrogen receptor-positive breast cancer. The approval marked the first targeted therapy for cancer, ushering in a new age of more precise and personalized approaches. Tamoxifen became one of the best-selling cancer drugs in the 1980s, following the results of large clinical trials that demonstrated its ability to significantly improve both disease-free survival and overall survival when used as an adjuvant therapy. The success of tamoxifen shifted the focus of drug discovery programs toward targeting specific molecules like growth factors and receptors, leading to the development of targeted therapies such as tyrosine kinase inhibitors and monoclonal antibodies. While these targeted therapies have proven to be effective against many cancers, they are not without challenges. Not all cancers have druggable targets, and tumors can develop resistance to these therapies over time. However, the promise of targeted therapy remains immense, as it offers more effective and less toxic treatment options.
Immunotherapy
Immunotherapy has emerged as one of the most exciting advancements in cancer treatment. Unlike therapies that directly target cancer cells, immunotherapy leverages the body’s immune system to recognize and destroy malignant cells. The concept of immunotherapy dates back to the late 19th century, when William Coley observed that some cancer patients experienced tumor regression after developing bacterial infections. Based on this observation, he began experimenting by injecting streptococcal bacteria into the tumors of inoperable cancer patients, presumably to stimulate the immune system to fight the cancer. After initially using live bacteria, which at times resulted in fatal outcomes, he then developed a safer mixture of heat-killed bacteria, which became known as Coley’s toxins. Despite the promising results, his approach, which was challenging to reproduce at that time, was overshadowed by the emergence of radiotherapy and chemotherapy that produced more consistent results. This field finally gained momentum in the 1990s with the discovery of immune checkpoint proteins. The inhibitory effects of cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) on the activation of T-cells were demonstrated by James Allison in 1995 and Tasuku Honjo in 1992, respectively. Following these breakthrough discoveries, ipilimumab, which targets CTLA-4 protein, became the first immune checkpoint inhibitor to reach the market in 2011 when it received the U.S. FDA approval for the treatment of metastatic melanoma. This was followed by the approval of pembrolizumab, a PD-1 inhibitor, in 2014 for advanced or unresectable melanoma. In 2017, pembrolizumab made history by becoming the first tumor-agnostic drug approved by the U.S. FDA. It was approved to treat any solid tumor with a specific genetic biomarker, marking a paradigm shift in cancer care. This landmark decision signified a move away from traditional treatment based on the tissue of origin, shifting the focus to the tumor’s molecular profile. In recent years, chimeric antigen receptor T-cell therapy (CAR T-cell therapy), a form of immunotherapy that involves genetically modifying a patient’s own T-cells to combat cancer more effectively, has emerged as another promising treatment option, particularly for patients with hematological malignancies. Although immunotherapy has shown remarkable success in treating advanced and previously resistant cancers, it remains a relatively new field. Further research is needed to understand why it is effective for some patients but not others. Additionally, efforts must be made to mitigate the risk of potentially life-threatening adverse events, which can occasionally accompany this form of therapy. The evolution of cancer treatment reflects the continuous advancement of medical science. From the rudimentary surgeries of ancient times to the state-of-the-art immunotherapies of today, each therapeutic approach has played a crucial role in improving patient outcomes and quality of life. While surgery, radiotherapy, and chemotherapy remain fundamental to cancer care, the rise of targeted therapy and immunotherapy marks a shift toward more personalized strategies. With on-going research and development, the next-generation of cancer therapies promises to be more precise and effective, with fewer side effects. Key innovations, such as cancer vaccines targeting tumor antigens, gene-editing technologies such as CRISPR for correcting mutations, and oncolytic virus therapy, offer new hope for transforming cancer from a once deadly disease into a more manageable, and potentially curable, disease in the near future.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.