Introduction
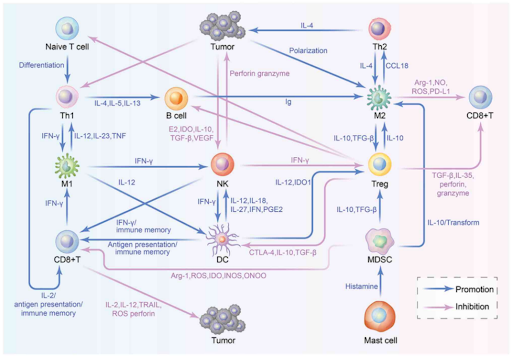
Tumor-infiltrating lymphocytes (TILs) are immune cells that infiltrate the tumor microenvironment and play an important role in the body’s defense against cancer. TILs, which include various types of immune cells such as T cells, B cells, macrophages and natural killer (NK) cells, can be broadly categorized into two categories based on their role in immune regulation. The first subset are immune cells that positively regulate immune response, such as CD4+ T helper 1 (Th1) cells, CD8+ cytotoxic T cells (CTLs) and NK cells, which can recognize, attack, and eliminate tumor cells. The second subset are immune cells that suppress the immune response, including tumor-associated macrophages (TAMs), regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), which secrete immunosuppressive factors, allowing tumor cells to escape immune surveillance and promote tumor growth.
Dual-role of TILs in cancers
-
Anti-tumor effect: TILs can recognize and target tumor cells, playing a crucial role in immune surveillance to prevent tumor development and metastasis. CTLs and NK cells, for example, are capable of directly killing tumor cells by recognizing specific antigens presented on the surface of the cancer cells. Additionally, these immune cells can secrete cytokines and other factors that stimulate a broader immune response to support tumor destruction. Thus, higher numbers of CTLs and NK cells in a tumor indicate better prognosis, as these cells can effectively reduce the tumor’s size.
-
Pro-tumor effect: In contrast, TILs can also contribute to tumor progression. For instance, Tregs and MDSCs can suppress anti-tumor response within tumor microenvironment by inhibiting the activity of CTLs, allowing tumor cells to escape immune surveillance. Besides that, TAMs are immune cells with two functionally distinct subtypes, with M1 phenotype exerting anti-tumor effects and M2 phenotype promoting tumor growth. Both phenotypes have a high degree of plasticity and can thus be converted into one another, upon changes in tumor environment or therapeutic interventions. In most tumors, the infiltrating macrophages are considered to be of the M2 phenotype, which release anti-inflammatory cytokines to promote cell proliferation, angiogenesis and tissue repair, providing an immunosuppressive environment for tumor growth.
TILs and immunotherapy
Despite the growing understanding of the role of TILs, there are still challenges in utilizing TILs effectively in immunotherapy. Tumors are often heterogenous, with some tumors being immunologically “hot” (having high levels of immune cell infiltration) and others being immunologically “cold” (lacking significant immune presence). Hence, a therapy that works well for one tumor may not necessarily be effective for another due to differences in immune response. Besides that, the tumor microenvironment is often immune suppressive, with immune checkpoints playing a crucial role in this suppression. TILs that infiltrate the tumor may become exhausted and become less effective at attacking tumor cells (known as immune exhaustion) due to excessive signaling of immune checkpoints such as PD-L1 and CTLA-4, which act as “brakes” on the immune system. Hence, immune checkpoint inhibitors such as pembrolizumab, nivolumab and ipilimumab, can help to boost anti-cancer immune response by enabling TILs to more effectively recognize and destroy cancer cells.
In recent years, adoptive cell therapy has emerged as a promising treatment. TIL therapy, which involves isolating TILs from a patient’s tumor, expanding them in the lab and then re-infusing them back into the patient, aims to boost the patient’s immune response in targeting and attacking tumor cells more effectively. On February 16, 2024, Amtagvi (lifileucel) was approved by the U.S. Food and Drug Administration (FDA) as the first cancer treatment using TILs. Amtagvi, a one-time, individualized T cell immunotherapy, is indicated for the treatment of adult patients with unresectable or metastatic melanoma previously treated with other therapies (a PD-1 blocking antibody, and if positive for the BRAF V600 mutation, a BRAF inhibitor with or without a MEK inhibitor). The accelerated approval was granted after a global, multicenter, multicohort clinical study demonstrated 31.5% objective response rate (ORR) in 73 patients who received Amtagvi, including three patients (4.1%) with a complete response and 20 patients (27.4%) with partial response. Among those who responded to the treatment, 56.5%, 47.8% and 43.5% continued to maintain responses without tumor progression or death at six, nine, and 12 months, respectively.
In summary, TILs play a key role in the response against cancer and can promote tumor progression or regression, with the outcome determined by the cellular composition of tumor microenvironment and interactions between cells residing in the tumor. Because these components undergo changes with tumor progression, the impact of TILs on the outcome can be high variable and unique in each cancer patient. Therefore, understanding how TILs function and interact with tumor microenvironment is vital to developing more effective and personalized therapies.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.