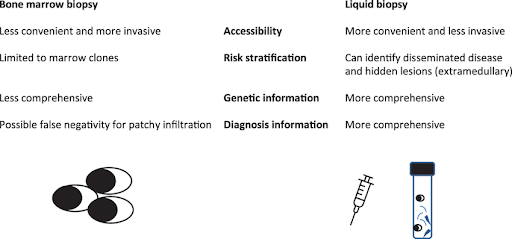
Bone marrow biopsy in hematological malignancies
Bone marrow biopsy is a medical procedure used to extract a small sample of bone marrow, the spongy tissue inside bones responsible for producing blood cells. This test provides valuable information to diagnose conditions such as leukemia or multiple myeloma, monitor treatment progression and disease relapse. The biopsy is usually done on the back of the hip bone (posterior iliac crest) under local anesthesia at the biopsy site, where a hollow needle is inserted through the skin into the bone to remove a small core of the marrow. This type of biopsy is referred to as a core biopsy because the needle removes a “core”, which is a cylinder-shaped tissue sample. Bone marrow biopsy is often accompanied by bone marrow aspiration, which involves removing a liquid sample from the bone marrow with a syringe attached to a needle for more detailed examination. The collected samples are then sent to a laboratory for analysis, where they are examined under a microscope to evaluate the number, type, and quality of blood cells. Additional tests such as flow cytometry and next-generation sequencing (NGS) may also be performed to further characterize the samples. While the procedure is generally regarded as low risk, possible complications may include bleeding, infection, bruising, and pain at the biopsy site, which can last for several days. Bone marrow biopsy can also be challenging to perform in patients with low platelet counts, those taking anticoagulants or antiplatelet medications (as they are at a higher risk of bleeding), or in those with a weakened immune system (since they are more susceptible to infections).
Liquid biopsy: a less invasive alternative to bone marrow biopsy
Liquid biopsy, which analyzes peripheral blood or other bodily fluids, offers a less invasive alternative to bone marrow biopsy with a lower risk of complications. By detecting circulating tumor DNA (ctDNA) shed from tumor cells into the bloodstream, liquid biopsy offers important insights into the genetic profile of the cancer, identify mutations that could guide treatment decisions, and monitor treatment response. Unlike bone marrow biopsy, which can be inconvenient and poses a higher risk, liquid biopsy provides a more accessible approach for longitudinal monitoring, making it especially useful in tracking minimal residual disease or identifying resistance mutations that may develop over time. Also, a single-site of bone marrow biopsy may create a sampling bias that provides a limited molecular profile as the biopsy may not represent all subclones. For example, multiple myeloma is a hematological malignancy characterized by a wide molecular heterogeneity even within the same patient, with the presence of subclones that differentially grow and evolve. Hence, ctDNA, which is more likely to reflect a wider range of subclones, including those from extramedullary sites, provides a more comprehensive and dynamic analysis of tumor profile compared to sampling from a single site. Importantly, liquid biopsy presents a valuable option for patients who are medically unfit to undergo bone marrow biopsy.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.