Next-Generation Genomic Profiling and Liquid Biopsy for Personalized Cancer Care
Canary Oncoceutics is a leading cancer diagnostics company that leverages cutting-edge genomics, artificial intelligence (AI), and machine learning (ML) to deliver highly sensitive and accurate tests. These innovative tests empower clinicians to make data-driven decisions and tailor treatment strategies based on the unique profile of each patient, driving better patient outcomes.
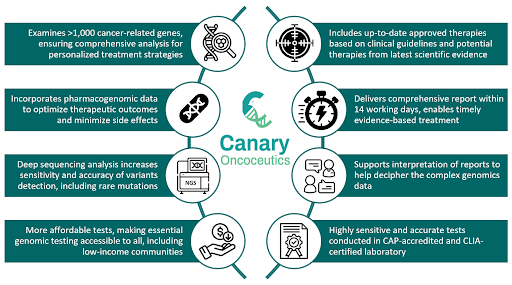
One of Canary’s flagship products is the Canary Acuity™, the most expansive comprehensive genomic profiling (CGP) test for solid tumors and hematological malignancies available on the market today. This test is designed to analyze single nucleotide variants, insertions, deletions, amplifications, and gene fusions across 1,091 cancer-related genes, along with genomic signatures like tumor mutational burden (TMB), microsatellite instability (MSI), and homologous recombination deficiency (HRD). It provides a comprehensive view of the tumor’s genetic landscape, identifying specific genetic alterations that can guide personalized treatment decisions, such as targeted therapies and immunotherapies, while also aiding in prognostication and diagnostic accuracy. By analyzing a broad panel of genes and multiple genetic signatures simultaneously, this approach significantly increases the likelihood of identifying actionable mutations immediately in a single test, avoiding delays and leading to more informed treatment strategies and better patient outcomes. Additionally, the inclusion of rare and emerging biomarkers, including dozens not found in other competing tests, could potentially open new treatment pathways or identify eligible clinical trials. Setting the Canary Acuity™ test apart from other commercially available tests is its integration of extensive germline analysis within the same assay. This includes all cancer-related genes that are reportable for secondary findings, as recommended by the American College of Medical Genetics and Genomics (ACMG), as well as pharmacogenomic variants. The inclusion of pharmacogenomic data is particularly important, as it helps clinicians understand not only which drugs could target a patient’s tumor more effectively, but also provides insights into the patient’s metabolism and response to a particular drug, maximizing treatment effectiveness while minimizing treatment-related side effects.
For patients where tissue biopsy is inaccessible or insufficient, Canary Pulse™ can serve as an alternative to tissue-based genomic testing. Tumors can release significant amounts of DNA fragments, known as circulating tumor DNA (ctDNA), into the bloodstream. Using the patient’s peripheral blood, this minimally invasive test interrogates 468 cancer-related genes for multiple classes of mutations, as well as including TMB, MSI, and HRD biomarkers, to guide treatment selection in solid tumors without the need for invasive tissue biopsies. Like Canary Acuity™, this test also provides extensive germline analysis. Since this test requires only a blood sample, Canary Pulse™ allows patients from remote or underserved regions without advanced healthcare facilities to also benefit from genomic testing. Additionally, this liquid biopsy test is especially useful in longitudinal monitoring to track disease progression, monitor treatment response, identify novel resistance mutations, and detect disease recurrence.
The integration of pharmacogenomic data in these tests is a distinguishing feature of Canary Oncoceutics. While many tumor profiling tests focus solely on identifying somatic mutations in the tumor, Canary Oncoceutics goes a step further by also analyzing germline variants involved in drug metabolism and response. This added layer of insight provides a more comprehensive approach, allowing therapy to be tailored to the patient’s genetic and tumor profile, which not only enhances treatment efficacy, but also improves the quality of life. Additionally, Canary Oncoceutics performs deep sequencing for its tumor profiling tests, with 2,000× coverage for tissue biopsy tests and 20,000× coverage for liquid biopsy tests, higher than most competing tests available on the market. Deeper sequencing boosts variant detection sensitivity, particularly for low-frequency variants, and improves accuracy by reducing false positives and false negatives. The capability to detect mutations, including rare mutations, with high confidence facilitates more accurate diagnoses and better- informed treatment options, as well as enabling earlier detection of early-stage cancers and potential relapses when the cancer burden is low.
Understanding the importance of rapid decision-making in cancer care, where timely decisions can directly impact treatment outcomes, Canary Oncoceutics ensures the delivery of high-quality, comprehensive reports within 14 business days from sample receipt. This commitment to swift turnaround time underscores the commitment to supporting clinicians with critical insights that enable timely, evidence-based interventions. Furthermore, these tests are conducted in laboratories accredited by College of American Pathologists (CAP) and certified by Clinical Laboratory Improvement Amendments (CLIA), ensuring that the results generated are not only accurate but also adhere to the highest standards of quality and reliability in clinical laboratory testing.
Both Canary Acuity™ and Canary Pulse™ tests provide crucial insights into a patient’s cancer, with the choice between them depending on several factors: the goal of the test, the patient’s overall condition, the type and stage of the cancer, as well as the sample types available for testing. Canary Acuity™ is ideal for obtaining a comprehensive information about the tumor’s molecular landscape and is particularly valuable for personalized treatment planning. On the other hand, Canary Pulse™, a minimally invasive test, offers a convenient method to monitor the disease in real-time, such as tracking disease progression, assessing treatment response, and detecting minimal residual disease. It is also the preferred option for patients who are medically unfit for invasive biopsy procedures, in cases where the tumor is too small or located in an area that is difficult or risky to access via a traditional tissue biopsy. Ultimately, combining both tests can provide a more comprehensive understanding of the disease, providing deeper insights at various stages of the treatment journey. This integrated approach allows clinicians to tailor treatment strategies more effectively and ensures on-going monitoring of treatment efficacy and potential disease recurrence.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.