Introduction
Prostate cancer is one of the most common cancers affecting men worldwide. As with all cancers, early detection is crucial for improving outcomes and reducing mortality rates. When caught and treated in its early stages, the prognosis for prostate cancer is typically favorable, with a five-year survival rate of over 99%. Because prostate cancer often develops slowly and may not exhibit symptoms at early stages, diagnostic tests play a pivotal role in its early detection. In line with its commitment to redefining early cancer detection and transforming patient care, Canary Oncoceutics has partnered with Pangia Biotech to launch CanaryScan™ Prostate, a urine-based diagnostic test aimed at the early prostate cancer detection.
Challenges of traditional prostate cancer detection tests
The blood-based prostate-specific antigen (PSA) test is one the primary tools widely employed in the detection of prostate cancer. Although the PSA test has played a central role in prostate cancer detection and helped improved outcomes for many patients, it is not an ideal tool. While a PSA level above 4 nanograms per milliliter (ng/mL) may indicate the presence prostate cancer, elevated levels can also be due to other benign conditions such as an enlarged prostate (benign prostate hyperplasia, or BPH) or inflammation (prostatitis). In contrast, it is not uncommon for men diagnosed with prostate cancer to have PSA levels within the normal range. The digital rectal examination (DRE) is another common tool for detecting prostate cancer. Besides being an invasive test (DRE works by assessing the prostate for abnormalities through the rectum), this test may miss smaller tumors or those that are located in areas not accessible during the examination. Even if abnormalities are felt, they do not necessarily indicate cancer, as other conditions like BPH or prostatitis can cause hard or irregular areas in the prostate. Additionally, DRE can be subjective as it largely relies on the examiner’s ability to palpate and interpret the feel of the prostate. The variability in skill among different examiners can lead to inconsistent results. Multiparametric magnetic resonance imaging (mpMRI) is an advanced MRI technique that combines multiple imaging sequences to provide a detailed picture of the prostate and its surrounding tissues. This helps to identify suspicious areas that may be cancerous and also assess the extent of the cancer more accurately. This non-invasive test has much higher sensitivity and specificity for detecting prostate cancer, particularly in men with elevated PSA levels or a suspicious DRE. However, its high cost, combined with the need for specialized imaging equipment and trained expertise, limits its accessibility, especially in low-resource areas. All these challenges can hinder or delay accurate results, resulting in missed diagnoses or unnecessary interventions.
CanaryScan™ Prostate transforming early prostate cancer detection
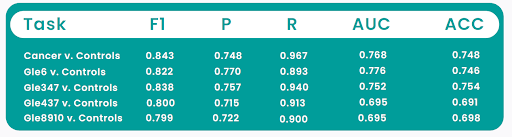
BCanaryScan™ Prostate offers a more sensitive, precise, and non-invasive approach to prostate cancer detection. By analyzing the biomolecule changes in a urine specimen using proprietary technologies and artificial intelligence (AI), this innovative test aims to improve early detection, reduce patient discomfort, avoid unnecessary biopsies, minimize missed diagnoses, and focus resources on patients who truly need further intervention. Findings from a recently concluded multicenter prospective study, presented at the American Society of Clinical Oncology (ASCO) 2025 meeting, showed that CanaryScan™ Prostate demonstrated superior performance, with an F1 score of 84.3%, a sensitivity of 96.7%, and a precision of 74.8%, in distinguishing patients with biopsy-positive prostate cancer from healthy controls. (Note: F1 score provides a more comprehensive evaluation than accuracy, especially in imbalanced datasets, by taking into account both false positives and false negatives). In addition to offering a more reliable and non-invasive diagnostic experience for patients, this test delivers accurate prostate cancer diagnoses with a rapid turnaround time, empowering clinicians to make timely, data-driven decisions, leading to better patient outcomes. With a simplified diagnostic process, CanaryScan™ Prostate promises to make cancer detection more accessible and affordable to all, benefiting both patients and healthcare systems globally. As technologies continue to evolve, Canary Oncoceutics is poised to shape the future of cancer diagnostics, helping to save lives and improve patient outcomes.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.