Introduction
Cancer treatment has undergone a tremendous evolution over the past few decades, from non-specific interventions such as chemotherapy and radiotherapy to more targeted and personalized therapies. In recent years, the chimeric antigen receptor T-cell therapy (CAR T-cell therapy) has emerged as one of the most exciting and revolutionary approaches to cancer treatment.
How CAR T-cell therapy works
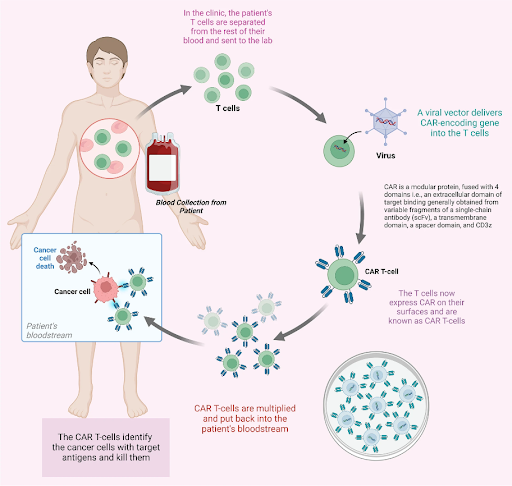
T cells (also known as T lymphocytes) are a vital component of the immune system, responsible for identifying and attacking foreign or abnormal cells such as bacteria, viruses, and cancer cells. Each T cell is equipped with a unique receptor that can bind to a specific antigen, a protein found on the surface of these cells, triggering a cascade of immune responses to eliminate these cells. CAR T-cell therapy is a form of immunotherapy that genetically modifies T cells to specifically target and destroy cancer cells. In CAR T-cell therapy, T cells are first collected from the patient’s blood through a process called leukapheresis. In the laboratory, these T cells are genetically engineered to express synthetic proteins known as CARs on their surface. These CARs are designed to recognize specific antigens found on the surface of cancer cells. The CAR T-cells are then grown and multiplied in the laboratory to create a sufficient dose for infusion. Before infusion, the cancer patient often undergoes lymphodepleting chemotherapy first to reduce endogenous immune cells in the body and create a microenvironment that is more conducive to the expansion and persistence of the CAR T-cells. After infusion, these engineered T cells seek out and eliminate cancer cells carrying the targeted antigens. Depending on the patient’s condition, bridging therapy can be administered after leukapheresis and before lymphodepleting chemotherapy, a period that can take several weeks. Bridging therapy, which may involve chemotherapy, radiotherapy and/or immunotherapy, aims to stabilize the disease progression or reduce tumor burden before CAR T-cells infusion.
Challenges of CAR T-cell therapy
CAR T-cell therapy has shown the most success in treating hematologic malignancies, where it has led to unprecedented remission rates, even in those with cancers that are relapsed or refractory to other treatments. The first CAR T-cell therapy was approved by the U.S. Food and Drug Administration (FDA) in 2017 to treat patients up to age 25 with acute lymphoblastic leukemia (ALL). Since then, more have been approved to treat pediatric and adult patients with blood cancers such as large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma. However, its effectiveness in solid tumors has been limited by various factors, such as antigenic diversity, an immunosuppressive tumor microenvironment, and dense tumor structures that impede CAR T-cells infiltration. Despite its transformative potential, CAR T-cell therapy can cause serious and life-threatening side effects. One of the most serious side effects is cytokine release syndrome (CRS), a condition where the infused CAR T-cells trigger a massive immune response that can lead to high fever, low blood pressure, and even organ failure. Another possible serious side effect is immune effector cell-associated neurotoxicity syndrome (ICANS), which can cause confusion, twitching, seizures, and loss of consciousness. Furthermore, CAR T-cell therapy requires highly personalized and complex procedures, which can take up to six weeks to manufacture a single patient’s dose. This extended production timeline makes the treatment less viable for patients with rapidly progressing disease. The limited manufacturing capability, combined with high costs and the need for specialized medical centers, further restricts accessibility for the majority of patients.
The future of CAR T-cell therapy
CAR T-cell therapy is a rapidly evolving field, with ongoing research focused on creating next-generation CAR T-cells designed to enhance cell infiltration and persistence within solid tumors, as well as overcoming tumor antigen escape by targeting multiple antigens on cancer cells. Efforts are also aimed at exploring new strategies such as combination therapies involving CAR T-cells alongside immune checkpoint inhibitors, to increase efficacy and reduce treatment-related toxicities. Additionally, work is underway to improve manufacturing processes and develop “off-the-shelf” allogeneic CAR T-cells, which could reduce both the waiting period and the costs associated with this therapy. While many hurdles remain, ranging from safety and efficacy to scalability and affordability, advancements in technology and cell engineering promise a future where CAR T-cell therapy could transform cancer treatment on a global scale.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.