Challenges and Strategies in Advancing CAR T-Cell Therapy
Over the last few years, chimeric antigen receptor T-cell therapy (CAR T-cell therapy) has revolutionized cancer therapy by demonstrating potent anti-tumor effects and high rates of durable responses, even in patients with relapsed or refractory diseases. To date, seven CAR T-cell therapies have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of various hematologic malignancies, such as acute lymphoblastic leukemia, large B-cell lymphoma, and multiple myeloma. However, several key challenges remain that limit the efficacy of CAR T-cell therapy and hinder its widespread adoption.
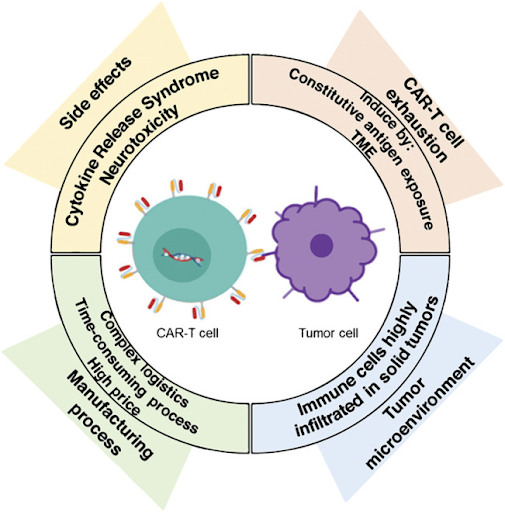
Toxicities:
Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are the most significant and frequently encountered complications of CAR T-cell therapy. It is estimated that CRS and ICANS occur in 42% to 90% and 20% to 60% of patients undergoing CAR T-cell therapy, respectively. The rapid activation and proliferation of CAR T-cells can trigger CRS through the overwhelming release of pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ), which can cause high fever, hypotension, hypoxia, and organ failure. While the exact mechanisms are still under investigation, ICANS is thought to result from a combination of factors, including a significant increase in pro-inflammatory cytokines, disruption of the blood-brain barrier, and potential infiltration of CAR T-cells into the central nervous system. Symptoms of neurotoxicity can range from headaches and confusion to memory loss, seizures, or even coma. High tumor burden and a large number of infused CAR T-cells are known to be significant risk factors for developing CAR T-cell associated toxicities. Strategies to manage these adverse events include early identification and grading of symptoms, as well as the use of IL-6 inhibitors like tocilizumab and/or corticosteroids to suppress the inflammatory cascade. Researchers are also exploring the incorporation of safety or suicide switches into the CAR structure that allows for deactivation of CAR T-cells if severe toxicity occurs, or fractionated dosing by administering CAR T-cells in smaller, multiple doses to prevent rapid expansion and activation.
Tumor heterogeneity and antigen escape:
Tumor heterogeneity poses a challenge in CAR T-cell therapy because the diverse expression of antigens across tumors means that CAR T-cells, which are typically engineered to target a single antigen, may fail to recognize and eliminate all cancer cells. This allows resistant cells to survive, leading to relapse. On the other hand, antigen escape occurs when tumor cells undergo genetic mutations or experience selective pressure during therapy that leads to the loss, alteration or downregulation of the target antigen, making them resistant to CAR T-cell recognition and killing. In some cases, tumors can mask the target antigen on their surface, making it inaccessible to the CAR T-cells, or undergo lineage switching (for example, from B-cell to myeloid), which eliminates the target antigen. All these mechanisms can lead to treatment failure or relapse. To address these challenges, several strategies have been developed to enhance the efficacy and durability of CAR T-cell therapy. Engineering CAR T-cells to recognize multiple antigens simultaneously or creating adaptive CAR T-cells that can respond to lower levels of the target antigen reduces the likelihood of tumor escape. Combinatorial strategies, such as sequential or combination treatments involving CAR T-cells targeting different antigens are also being explored. Another innovative approach is to promote epitope spreading by co-treating CAR T-cells with immunomodulators like IL-12, which enhance the activation of antigen-presenting cells (APCs). Epitope spreading works by inducing a broader immune response beyond the initially targeted antigen. The killing of the tumor cells by the CAR T-cells releases tumor debris containing new antigens that are processed and presented by the APCs, thereby activating endogenous T-cells against these secondary antigens.
Tumor microenvironment:
Unlike blood cancers, solid tumors possess a complex and immunosuppressive microenvironment that hinders CAR T-cell infiltration while contributing to its exhaustion. Key components include immunosuppressive cells such as myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), soluble factors like cytokines and prostaglandins, and immune checkpoints like PD-L1, which inhibit CAR T-cell anti-tumor effects and proliferation. Also, physical barriers such as dense stromal tissue and disorganized tumor vasculature impede CAR T-cell trafficking and infiltration. Hypoxic and acidic conditions, along with nutrient scarcity, within the tumor microenvironment further negatively affect CAR T-cell proliferation and survival. Strategies to overcome these challenges include engineering CAR T-cells to secrete factors that suppress immunosuppressive cells like regulatory T-cells, or to secrete pro-inflammatory cytokines like IL-12 and IL-15, which promote a more favorable tumor microenvironment. Combining CAR T-cell therapy with immune checkpoint inhibitors or oncolytic viruses may also improve its efficacy in different cancers.
Manufacturing process:
The process of collecting, engineering, expanding, and reinfusing autologous CAR T-cells is complex, time-consuming, and expensive. This individualized approach can delay treatment for critically ill patients and is not always feasible for those with insufficient T-cell counts. Collecting T-cells earlier in the patient’s treatment regimen, before their immune system is compromised by extensive therapy, can improve the quality and consistency of starting material. In vivo CAR T-cell engineering is an innovative approach that engineers CAR T-cells inside the patient’s body. While there are hurdles with this approach such as vector specificity, potential off-target effects, and concerns about long-term efficacy and safety, it bypasses the costly, lengthy, and laborious ex vivo manufacturing process. This could lead to simpler logistics, reduced costs, improved efficiency, and better patient outcomes. Efforts are also being made to develop allogeneic (off-the-shelf) CAR T-cells derived from healthy donors, which allows for standardized, large-scale production to reduce the high costs and manufacturing time associated with patient-derived CAR T-cells. Although using T-cells from a healthy donor increases the risk of graft-versus-host-disease (GvHD) and host rejection, gene editing tools like CRISPR can be utilized to modify donor T-cells to reduce the risk of immune rejection and improve the safety of these allogeneic CAR T-cells.
Undoubtedly, CAR T-cell therapy has transformed the cancer treatment landscape by providing targeted, potent, and potentially long-lasting immunity, particularly for certain relapsed or refractory hematological malignancies. However, several key biological and technical issues remain. The risk of severe, life-threatening toxicities underscores the need for effective mitigation strategies. Additionally, issues such as tumor heterogeneity and antigen escape, both of which can lead to treatment resistance, as well as the immunosuppressive tumor microenvironment and physical barriers in solid tumors, must be addressed. The lengthy and costly manufacturing process also presents a significant obstacle, hindering timely access to this life-saving therapy. To overcome these challenges, researchers are focused on refining CAR T-cell design for more precise targeting with a better safety profile, improving manufacturing efficiency, and exploring combination therapies to increase efficacy while minimizing adverse effects. Other emerging strategies include reprogramming the tumor microenvironment, improving delivery mechanisms, developing in vivo CAR T-cells, and creating allogeneic CAR T-cells. As these innovations progress, CAR T-cell therapy holds the potential to become a safer, more effective, and widely accessible treatment option for a broader range of cancers in the future.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.