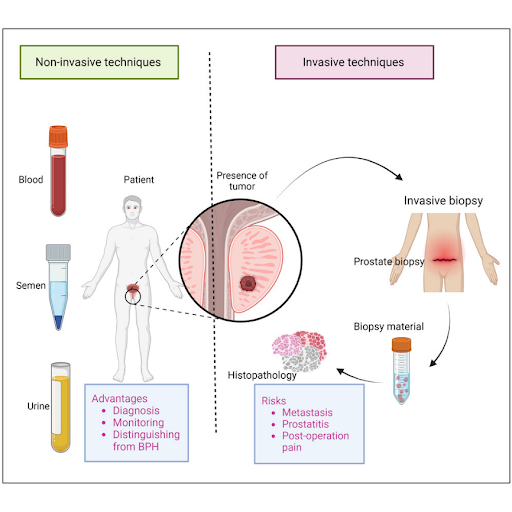
Introduction
The prostate-specific antigen (PSA) test is commonly used to screen for prostate cancer by measuring the level of PSA, a protein produced by the prostate gland, in the blood. While the total PSA test has been widely used for decades, it has limitations in distinguishing between prostate cancer and benign conditions. Typically, a PSA level below 4 nanograms per milliliter (ng/mL) is considered within the normal range, while a level above 10 ng/mL significantly increases the likelihood of prostate cancer and generally necessitates a prostate biopsy to confirm or rule out cancer. On the other hand, a PSA level between 4 and 10 ng/mL is often referred to as the “gray zone” as results in this range are not clearly indicative of prostate cancer, and there is no clear consensus on what PSA level warrants proceeding with a prostate biopsy. To improve the accuracy of prostate cancer detection and risk stratification, especially for those with PSA levels in the gray zone, many variations of PSA tests have been developed over the years.
Age-adjusted PSA:
PSA levels tend to increase naturally with age, due to prostate enlargement and becoming more permeable with age. Hence, older men usually have higher levels of PSA than younger men, even without prostate cancer or other benign conditions. Therefore, it may be more appropriate to have age-specific reference ranges, instead of relying on a single reference range for men of all age groups. Research has previously recommended the following reference range for serum PSA (95th percentile): 0.0 to 2.5 ng/mL for men aged 40 to 49 years; 0.0 to 3.5 ng/mL for men aged 50 to 59 years; 0.0 to 4.5 ng/mL for men aged 60 to 69 years; and 0.0 to 6.5 ng/mL for men aged 70 to 79 years. However, using age-adjusted PSA ranges has not been proven effective in consistently identifying clinically significant cancers while minimizing unnecessary biopsies.
Percentage of free PSA:
Two forms of PSA are present in the blood: one form is attached to proteins, while the other circulates free and unattached. The amount of free PSA compared to the total PSA is referred to as the percentage of free PSA. A lower percentage of free PSA suggests a higher likelihood of prostate cancer, suggesting that a biopsy should be performed. Typically, a cut-off of 10% or lower for percentage of free PSA prompts a biopsy, while a biopsy should also be considered if the value falls between 10 and 20%. However, experts disagree on the optimal cut-off value for determining when a biopsy is necessary, and this may vary depending on the initial PSA level.
PSA velocity:
This refers to the change in PSA levels over time. Since PSA levels gradually increase with age, a rapid rise in PSA levels may indicate the presence of prostate cancer or an aggressive form of cancer. However, it should be noted that the sudden increase in PSA levels can also be caused by other conditions, such as inflammation or infection. Recent studies indicate that PSA velocity has limited clinical value and adding it to the standard risk models does not improve accuracy of prostate cancer detection.
PSA density:
This accounts for higher PSA levels in men who have larger prostates. The prostate volume is measured using magnetic resonance imaging (MRI) or transrectal ultrasound, and the PSA value is then divided by the prostate volume to obtain the PSA density. Since prostate cancers can produce more PSA per volume of tissue than benign conditions, a PSA density of 0.15 ng/mL/cc or more is indicative of prostate cancer. While PSA density can provide valuable insights, it relies on accurate prostate volume measurements, which can vary depending on the method used. Additionally, although 0.15 ng/mL/cc is often used as the threshold, the optimal PSA density cut-off for guiding prostate biopsy decisions has not been definitively established and may differ according to the clinical context. Although PSA screening is part of the standard of care, its limitations have spurred the development of various enhancements aimed at improving diagnostic accuracy. While these advancements in PSA testing have allowed for a more individualized approach to prostate cancer screening, none has yet demonstrated that the PSA test alone can serve as standalone decision-making tool for guiding prostate biopsies. As a result, ongoing research is increasingly focusing on exploring new biomarkers, novel approaches, and alternative sample types to establish a more standardized method for assessing prostate cancer risk. These efforts aim to detect prostate cancers at an earlier stage with greater sensitivity and specificity, using less invasive diagnostic methods to improve patient outcomes and minimize unnecessary biopsies.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.