Decoding the Genetic Blueprint of Cancer with Germline and Somatic Testing
Genetic testing plays a crucial role in cancer risk assessment, diagnosis, prognosis, and treatment. There are two main types of genetic testing in cancer: germline and somatic testing, each serving a different but complementary purpose. Understanding the distinction between these two approaches is essen
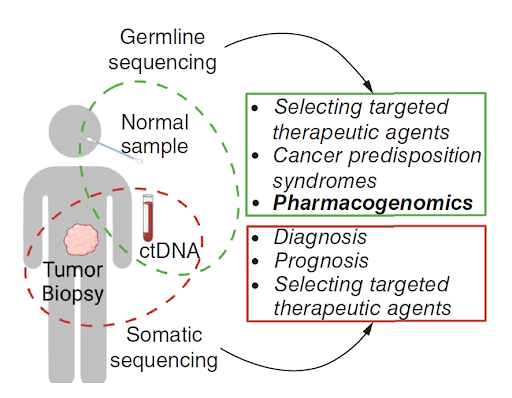
Germline testing in cancer care
Germline testing, which looks for inherited mutations that are present in all cells of the body from birth, can be performed using various sample types, including blood, saliva, and cheek swabs. These mutations are heritable (passed from one generation to the next) and may be found in multiple family members. Findings from germline testing can influence the prevention and screening strategies for both individuals and their family members. For example, women at higher risk of developing breast cancer due to mutations in BRCA genes may consider preventive measures such as surgical options (e.g., prophylactic mastectomy) or chemoprevention (tamoxifen and raloxifene are US FDA-approved drugs for breast cancer risk reduction), as well as surveillance strategies like starting screening at an earlier age and having more frequent screenings to detect cancer early when it is more treatable. Since the results of germline testing reflect inherited mutations, they may prompt at-risk family members to seek genetic testing for themselves. While germline testing is typically done to assess cancer risk in individuals, it can also influence treatment decisions in cancer patients. Studies have shown that PARP inhibitors like olaparib are more effective in patients with germline BRCA mutations. Additionally, these patients may also be more responsive to platinum-based chemotherapy. Germline testing can also provide information on pharmacogenomic variants, which can guide optimal drug selection and dosing to maximize drug efficacy and minimize drug-related toxicity. Although the associations between pharmacogenomic variants and anticancer drugs are well-supported by evidence (e.g., DPYD with fluoropyrimidines and UGT1A1 with irinotecan), the adoption of pharmacogenomic testing continues to lag behind disease testing in clinical practice, despite its potential to improve personalized cancer treatment.
Somatic testing in cancer care
Somatic testing detects acquired mutations in cancer cells, typically using tissue biopsies or liquid biopsies as sample types. Because these mutations arise from environmental exposures (e.g., tobacco smoke, radiation, or UV light), aging, or random cellular events (e.g., errors in DNA replication), they are present only in the tumor and not in all cells of the body. The goal of somatic testing is to identify actionable mutations that can be used to diagnose specific types of cancer, determine prognosis, and guide treatment decisions. For instance, lung cancer patients with EGFR mutations can be treated with tyrosine kinase inhibitors such as erlotinib or osimertinib, which leads to better outcomes compared to a one-size-fits-all chemotherapy approach. Unlike germline testing, adoption of somatic testing has grown exponentially in clinical practice, driven by the increasing number of molecularly targeted therapies approved by regulatory bodies and guidelines from professional organizations like National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), and European Society of Medical Oncology (ESMO). As the number of targeted therapies expand, next-generation sequencing (NGS) has emerged as an indispensable tool in matching patients with the most effective, tailored treatment options based on a tumor’s unique genetic makeup. In summary, germline testing is used to assess inherited cancer risk, select targeted therapies, and guide drug selection and dosing, whereas somatic testing is used for diagnosis, prognostication, and the selection of targeted therapies. Integrating both germline and somatic testing into clinical practice allows for more precise and personalized cancer care, enabling tailored treatment strategies, better assessment of cancer predisposition, and more effective preventive measures for patients and their families. This comprehensive approach not only enhances early detection and intervention but also improves treatment efficacy, leading to better survival rates and quality of life.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.