Unlocking the Success of Immune Checkpoint Inhibitors with Predictive Biomarkers
Immune checkpoint inhibitors (ICIs) have become a game-changer in cancer treatment, offering new hope to patients with cancers that were previously difficult to treat. These therapies work by blocking immune checkpoint proteins that prevent T-cells from attacking cancer cells, leading to significant improvements in patient survival and quality of life. However, not all patients benefit equally from these ICIs. Hence, identifying predictive biomarkers has become crucial for determining which patients are most likely to respond to ICIs. This personalized approach not only minimizes unnecessary side effects but also helps optimize treatment outcomes.
Immune checkpoints and cancer
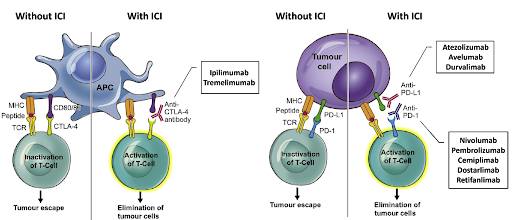
Immune checkpoint proteins are regulatory proteins that act like switches, preventing immune cells from attacking normal, healthy cells. The programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) are the two most well-known immune checkpoint proteins that play an important role in tumor immune evasion. Under normal conditions, these proteins act as negative regulators of immune responses, preventing excessive inflammation and autoimmunity. However, many cancer cells exploit these proteins to evade detection and destruction by the immune system. Cancer cells can express ligands such as programmed death-ligand 1 (PD-L1), which binds to PD-1 on T-cells and inhibit their activity, allowing the tumor to evade immune detection and grow unchecked. Additionally, tumor cells can release signals that promote the expression of CTLA-4 and lymphocyte activation gene-3 (LAG-3) on T-cells, leading to immune suppression and further hindering the anti-tumor immune response. ICIs are designed to block these immune checkpoint proteins, enabling the T-cells to recognize and attack cancer cells more effectively. To date, many ICIs have been approved by the U.S. Food and Drug Administration (FDA) for various cancers, such as PD-1 inhibitors (nivolumab, pembrolizumab, cemiplimab, dostarlimab, and retifanlimab), PD-L1 inhibitors (atezolizumab, avelumab, and durvalimab), CTLA-4 inhibitors (ipilimumab and tremelimumab), and LAG-3 inhibitor (relatlimab).
Biomarkers are crucial in driving the efficacy of ICIs
ICIs have shown remarkable success in treating various cancers, such as melanoma and non-small cell lung cancer. However, not everyone will benefit from these therapies, and the outcomes can be less favorable for some patients. While some patients may experience durable responses and even complete remission, others may suffer severe immune-related adverse effects like myocarditis and pneumonitis, which could be fatal and outweigh any potential therapeutic benefit. This variability underscores the importance of predictive biomarkers and personalized treatment approaches to identify patients who are most likely to respond to ICIs, while avoiding unnecessary harm to those who would not benefit.
PD-L1 expression is most well-established biomarker for predicting response to PD-1/PD-L1 inhibitors. Testing for PD-L1 expression is performed through immunohistochemistry (IHC), a laboratory technique that uses antibodies to detect PD-L1 on tumor tissue samples. Tumors that express higher levels of PD-L1 expression have been correlated with a better response to PD-1/PD-L1 inhibitors. However, it is not a perfect predictor. Some patients with low or absent PD-L1 expression can still benefit from the therapy, while others with high expression may not.
Tumor mutational burden (TMB) refers to the total number of mutations present in the tumor’s genome and is expressed as the number of mutations per megabase (mut/Mb). TMB can be measured using next-generation sequencing (NGS), and tumors with a TMB score of ≥10 mut/Mb are generally considered as TMB-High (TMB-H). TMB-H tumors are likely to produce more neoantigens, which are recognized as foreign by the immune system, leading to a stronger immune response against the tumor. Therefore, higher TMB score is often associated with a better response to ICIs, as the immune system has more targets to attack. However, it should be noted that not all patients with high TMB respond to ICIs, and vice-versa. Other factors, such as immune cell infiltration and tumor microenvironment can also influence the response to ICIs.
Microsatellite instability (MSI) is caused by the deficiency of the DNA mismatch repair (MMR) system, leading to the accumulation of mutations in the repetitive DNA regions. MSI can be assessed using polymerase chain reaction (PCR) or NGS to detect instability in the microsatellite regions, while IHC can be used to detect the presence or absence of key MMR proteins, such as MLH1, MSH2, MSH6, and PMS2 in tumor tissue samples. Tumors with high MSI (MSI-H) or deficient MMR genes have shown higher response rates to ICIs. While they are strong predictive biomarkers for ICI response, their prevalence varies significantly across different cancers, occurring in 20-40% of endometrial cancers, 10-15% of colorectal cancers, 10-30% of small bowel cancers, 10% in gastroesophageal cancers, and approximately 4% in other solid tumors.
Tumor-infiltrating lymphocytes (TILs) are immune cells that have infiltrated the tumor microenvironment, indicating an active immune response against the tumor. Although TILs are typically assessed using visual scoring on hematoxylin and eosin (H&E) stained slides, flow cytometry can be used to characterize the phenotype and function of TILs for a more detailed analysis. Additionally, newer methods like NGS enable more in-depth characterization of TILs by measuring their RNA expression or performing T-cell receptor (TCR) sequencing. While not yet a standard clinical practice, TILs have emerged as a promising biomarker for predicting response to ICIs, as their presence in a tumor (particularly CD8+ T-cells) is often associated with a better response to the ICIs, particularly in certain cancers such as melanoma and breast cancer.
The need for more accurate predictive models for better outcomes
ICIs have shown the potential for durable, long-term responses in patients with advanced cancers, including those with limited treatment options. However, the response to ICIs can be inconsistent, with some patients experiencing significant benefits, while others show little to no response. Biomarkers such as PD-L1 expression, TMB, and MSI are commonly used to guide treatment decisions, helping to personalize care, avoid ineffective treatments, and improve patient outcomes. While these biomarkers provide useful insights, their predictive power is not absolute. For instance, not all TMB-H or MSI-H tumors respond uniformly to ICIs, indicating that relying solely on individual biomarkers may not paint a complete picture. Hence, there is a concerted effort to develop more refined and comprehensive biomarkers, including multi-biomarker strategies, that can better predict treatment outcomes. Biomarkers that take into account not just the tumor characteristics, but also the patient’s immune system and genetic profile could increase the overall success rate of ICIs, reduce the risk of adverse effects, and spare patients from ineffective treatments that may cause more harm. Importantly, comprehensive genomic profiling (CGP) has emerged as a highly sensitive, cost-effective, and tissue-preserving alternative to serial single-biomarker tests. Besides enabling a broad analysis, CGP typically incorporates established biomarkers such as TMB and MSI, along with emerging biomarkers like TILs, within a single assay. This approach provides a more holistic view of the patient’s potential responsiveness to ICIs, empowering clinicians to make more informed treatment decisions, optimize therapeutic outcomes, and enhance the quality of life for cancer patients.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.