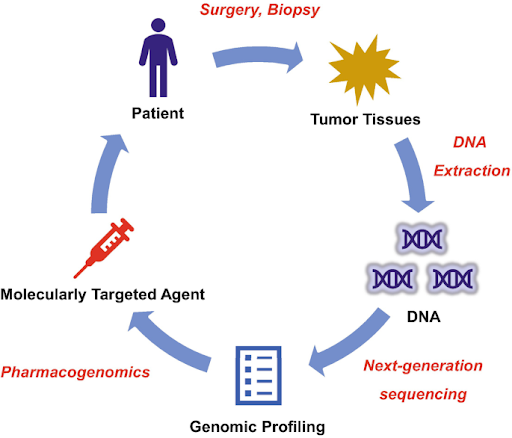
Improving treatment outcomes through genomic profiling
Most cancers are sporadic and arise from somatic mutations that accumulate over an individual’s lifetime, often due to environmental factors, aging, and errors in DNA replication and repair. These mutations are unique to each tumor and can differ significantly between cancer patients, even those with the same cancer type. Genomic profiling involves analyzing genetic alterations in the tumor to identify actionable mutations that can inform targeted therapy choices. For example, non-small cell lung cancer patients with EGFR mutations may benefit from EGFR inhibitors such as gefitinib and erlotinib, which can block the activation of the EGFR pathway which drives cancer growth and progression. On the other hand, KRAS mutations in colorectal cancer are known to be a key predictor of resistance to EGFR inhibitors like cetuximab and panitumumab. These targeted therapies have transformed treatment outcomes by offering a more precise approach with fewer side effects than traditional chemotherapy, making genomic profiling an integral part of cancer diagnosis and treatment.
Optimizing therapeutic success with pharmacogenomics
Pharmacogenomics is a branch of personalized medicine that studies how genetic differences among individuals affect their responses to drugs. It combines pharmacology (the study of drugs) and genomics (the study of genes and their functions) to elucidate how genetic variations influence drug metabolism, efficacy, and safety. Besides environmental factors such as diet and lifestyle, genetic variations can have a significant impact on how medications are processed in the body. Inherited (or germline) mutations can affect the pharmacokinetics (absorption, distribution, metabolism, and elimination of drugs) and pharmacodynamics (relationship between drug concentration and its effect on the body) of a treatment, which may in turn impact the individual’s response to a particular drug. For instance, genetic variations in drug-metabolizing enzymes such as cytochrome P450 (e.g., CYP2D6 and CYP3A4) can affect the metabolism rate of certain drugs. Individuals with certain variants may require higher or lower doses to achieve optimal therapeutic effect while avoiding toxicities. On the other hand, certain genetic variants could lead to resistance to specific drugs, rendering the drugs ineffective. By analyzing the DNA sequence of specific genes involved in drug metabolism and response, this approach enhances the likelihood of positive treatment outcomes while minimizing the risk of adverse reactions.
Next-generation sequencing empowers more comprehensive approach
In recent years, next-generation sequencing (NGS) has emerged as a rapid and cost-effective method for high-throughput analysis of multiple genes in a single assay, allowing simultaneous analysis of both tumor mutation profiles and pharmacogenomic data for more informed treatment decisions. Integrating pharmacogenomics with genomic profiling significantly enhances the precision and effectiveness of cancer treatment, enabling individualized therapy based on the unique genetic makeup of both the patient and the tumor. For example, genomic profiling identified EGFR exon 21 (L858R) substitution mutation in a non-small cell lung cancer patient, suggesting that the patient may benefit from tyrosine kinase inhibitors such as gefitinib. However, it was revealed concurrently that the same patient also carries a CYP2D6 poor metabolizer variant, indicating that the patient may not metabolize gefitinib efficiently compared to general population. According to the U.S. Food and Drug Administration (FDA), higher systemic concentrations of the drug and higher adverse reaction risk may be observed in CYP2D6 poor metabolizer patients taking gefitinib. While there is no dose modification recommended for this group of patients, close monitoring for adverse reactions such as hepatotoxicity and severe rashes is essential. Alternatively, other similar targeted therapies such as erlotinib and osimertinib (CYP2D6 plays a less significant role in the metabolism of these two drugs) can also be considered for this group of patients. This comprehensive approach, whereby genomic profiling is used to guide therapy selection while pharmacogenomics is used to personalize dosing and monitoring, helps to optimize treatment efficacy and minimize toxicity, leading to better patient outcomes.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.