Understanding MRD in cancer recurrence
Minimal residual disease (MRD; also known as measurable or molecular residual disease) refers to the small number of cancer cells that persist in the patient’s body after local or systemic cancer treatment. Residual cancer cells after treatment can occur due to several reasons such as incomplete elimination of all cancer cells, the presence of dormant or resistant cells, and the development of drug resistance through clonal evolution. Although the number of remaining cancer cells is often too small to be detected by standard laboratory tests or traditional imaging scans immediately, they can multiply rapidly and lead to a relapse. MRD monitoring has traditionally been used in hematological malignancies, such as leukemia and multiple myeloma, where trace amounts of cancerous cells can sometimes be detected in the blood or bone marrow even after treatment. Over time, rapid technological innovations have expanded MRD testing to other cancer types, including solid tumors, thereby enhancing its role in precision oncology.
Detecting MRD in cancers: from phenotypic to molecular assays
The key challenge of detecting MRD lies in its ability to remain “hidden” in the body, making it an invisible but potent threat that can re-emerge later. Hence, it is not surprising that these residual cancer cells play a pivotal role behind the majority of cancer recurrences. The accurate identification of MRD has become a cornerstone in precision oncology, with significant implications in prognostics, treatment decisions, and relapse prevention. The most widely used assays for MRD detection are flow cytometry, polymerase chain reaction and next-generation sequencing.
Flow cytometry:
Multiparameter flow cytometry remains one of the most widely used methods to detect MRD, especially in hematologic malignancies. It involves the use of fluorescently-labeled antibodies to identify abnormal cell populations based on their surface markers. Although it is highly specific, this assay has limited sensitivity (10-4 to 10-5 sensitivity) and requires skilled interpretation.
Polymerase chain reaction (PCR):
Since PCR amplifies specific DNA or RNA sequences for detection, it provides a more sensitive approach (10-5 to 10-6 sensitivity) in detecting MRD. Digital droplet PCR (ddPCR), in particular, enhances this precision and sensitivity by partitioning samples into thousands of droplets, each undergoing an individual PCR reaction. Hence, ddPCR has been shown to be highly accurate for tracking MRD in a variety of cancers. However, the drawbacks of this approach include the need for known targets and a limited multiplexing capability.
Next-generation sequencing (NGS):
NGS has revolutionized MRD detection, by enabling ultra-deep sequencing of tumor-specific alterations for increased specificity and sensitivity (up to 10-6 sensitivity). Unlike flow cytometry and PCR, NGS offers unbiased detection of a broad range of mutations, has high multiplexing capability and does not require prior knowledge of targets, providing a more comprehensive approach. Despite having many advantages, its widespread adoption is limited by higher cost, longer turnaround time and the need for more sophisticated infrastructure compared to flow cytometry and PCR assays.
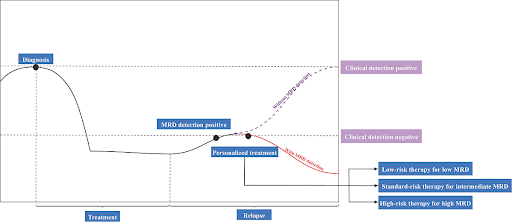
Clinical significance of MRD in cancer management
A positive MRD test result means that there are still residual cancer cells in the body after treatment, indicating that the treatment was not completely effective. On the other hand, a negative MRD test result means that no trace of cancer cells was found in the body after treatment. Multiple studies have shown that patients who are MRD-negative after treatment have a much lower risk of relapse and better outcomes compared to those who remain MRD-positive after treatment. Hence, MRD status plays a key factor in risk stratification, helping to identify patients at higher risk of relapse who may benefit from more intensive treatment or closer follow-up. Also, MRD information can be used to assess treatment response and guide adjuvant therapy decisions, allowing for adjustments in treatment intensity and duration, or change in the treatment strategy. MRD tests can also be used for longitudinal monitoring to detect early signs of relapse – months before the disease becomes clinically or radiographically evident – potentially allowing for earlier intervention and better patient outcomes. The landscape of MRD detection is rapidly evolving. While hematologic cancers have long benefitted from MRD monitoring, technological advancements and AI-driven bioinformatics have opened new frontiers for its application in solid tumors. Continued research and clinical validation will be critical to integrate MRD monitoring into clinical practice across a broad spectrum of cancers to improve patient stratification, optimize treatment regimens, and ultimately maximize long-term outcomes in the era of precision oncology.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.