Critical Steps in Advancing Medical Research
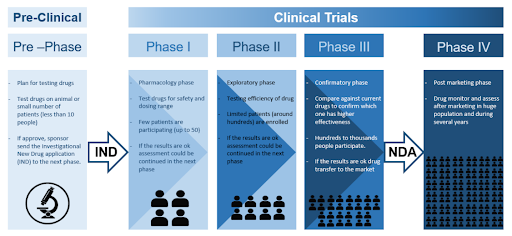
Clinical trials are research studies designed to evaluate new or existing treatments, devices or procedures for detecting, managing and preventing diseases. These trials aim to provide insights into the safety and effectiveness of new interventions by comparing them to the current standard of care. They are essential in driving medical innovations, especially in drug development for serious diseases like cancer. Clinical trials must be completed and evaluated before new treatments can be approved by regulatory authorities such as the U.S. Food and Drug Administration (FDA).
-
Anti-tumor effect: Clinical trials are only conducted after pre-clinical findings suggest that the new drug or treatment is likely to be safe and effective. Cell studies, which involve testing on laboratory-grown cells, are often the first tests done in pre-clinical studies. Promising treatments are then tested in live experimental animals to further evaluate their efficacy and safety. Though valuable, pre-clinical studies have limitations as differences between humans and small animals can affect how a treatment works. Additionally, side effects or other issues not observed in small animal studies may arise in humans.
-
Phase 0 trials: Phase 0 trials are sometimes conducted under an exploratory Investigational New Drug (eIND) to support clinical evaluation before the dose escalation, safety and tolerance studies associated with traditional IND. These trials have no therapeutic intent and involve only a small group of patients or healthy volunteers who receive a low (sub-therapeutic) dose of the drug for a short duration. The goals of phase 0 trials include assessing the modulation of a presumed drug target in humans, optimizing target assay methodology, obtaining pharmacokinetic data, evaluating pharmacokinetic/pharmacodynamic relationships, and identifying the most promising lead agent from various chemical entities or formulations. Although phase 0 trials are not mandatory for an IND application, they can help to provide early insights, reduce uncertainties and improve the likelihood of success in later trials.
-
IND application: Before a clinical trial can be started, a drug sponsor (typically a pharmaceutical company) must submit an IND application to the regulatory authorities. This application includes detailed information about the drug’s composition, manufacturing process, pre-clinical data, study protocols, and details about the clinical trial team. The clinical trial can only commence after the regulatory authorities grant clearance of the IND application.
-
Phase 1 trials: This is the first phase of clinical trials, primarily aimed at determining the safety of a new drug. Although only a small group of participants (usually less than 20) are recruited at this phase, phase 1 trials can take a long time to be completed because participants are slowly recruited for dose escalation studies (placebos are typically not used at this phase). The trial starts with low doses for the first few participants and gradually increases as participants are closely monitored. This process continues until an optimal dose with an acceptable safety profile is identified. At the same time, the pharmacokinetics and pharmacodynamics data are also collected. Since phase 1 trials carry the most potential risks, participants are usually those with advanced diseases who have exhausted other treatment options. While most participants are not expected to benefit from this trial, some participants may.
-
Phase 2 trials: Phase 2 trials are usually randomized studies focusing on the drug’s efficacy and safety for a specific condition or disease in a relatively homogenous patient population. The primary goal of phase 2 trials is to assess whether the drug has the desired therapeutic effect, by comparing the new drug with another treatment already in use or a placebo. Concurrently, the optimal dose and treatment regimen are also determined at this phase. Since phase 2 trials involve a larger number of participants (typically hundreds) undergoing treatment over a longer duration than phase 1 trials, less common and longer-term side effects may be observed.
-
Phase 3 trials: Before the drug can be approved for use by the regulatory authorities, the drug must confirm its efficacy, safety and benefit-risk profile for the intended indication in phase 3 trials. Phase 3 trials are often randomized, multicenter trials conducted in different hospitals (and even different countries) involving hundreds to thousands of participants. The main goal of phase 3 trials is to demonstrate that the new treatment leads to better patient outcomes compared to current standard of care. Unless there is no treatment currently available, placebos are generally not used in phase 3 trials. The bulk of the information required for the package insert and labelling of a drug, after it is approved by the regulatory authorities, comes from the data generated during phase 3 trials. If the drug demonstrates significant clinical benefits with an acceptable safety profile in phase 3 trials, the drug sponsor can submit a New Drug Application (NDA) to regulatory authorities for review. Based on the data submitted, the regulatory authorities can either approve the drug to be marketed, request more information, or even require that more studies be done.
-
Phase 4 trials: After the drug has been approved and marketed in the general population, post marketing surveillance continues under phase 4 trials. The objectives of phase 4 trials are to collect real-world data, monitor long-term effectiveness, and observe for potential rare or long-term side effects in broader, more diverse populations for a period of time (usually several years). This ensures continuous assessment of the overall benefit-risk profile of the drug. Phase 4 trials may sometimes uncover new information on the drug such as new drug interactions or rare side effects not observed in earlier phases, which can lead to updates to the drug’s labelling (new warnings, precautions or contraindications). In rare cases, phase 4 trials may also reveal serious risks that were not detected earlier. If these risks outweigh the benefits, regulatory authorities may restrict the use of this drug or withdraw the drug from the market.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.