The Role of Prostate-Specific Antigen in Prostate Cancer Screening
Prostate cancer is one of the most common cancers affecting men worldwide, particularly those over the age of 50. Prostate-specific antigen (PSA) is a protein produced by the prostate, a small gland that sits below the bladder in men. The PSA test, which measures the level of PSA in the blood, is one of the primary tools used in the early detection and monitoring of prostate cancer. However, it’s utility is suboptimal due to its low specificity, and interpreting the results can be complex due to the various factors that can influence PSA levels.
PSA levels can be influenced by prostate cancer but also other conditions
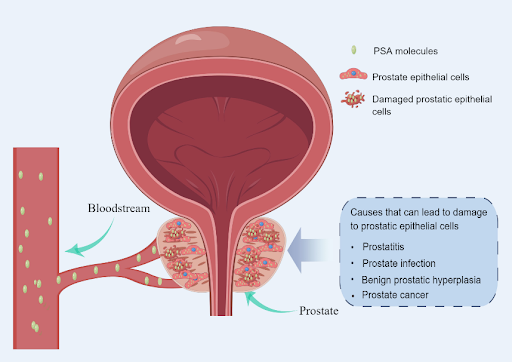
While PSA is primarily found in semen, which is also produced in the prostate, a small amount of PSA can be detected in the bloodstream of healthy individuals. PSA levels are typically below 4 nanograms per milliliter (ng/mL) of blood in healthy men, although younger men generally have lower levels. Elevated PSA levels may indicate the presence of prostate cancer, prompting a prostate biopsy. This is because when prostate epithelial cells are damaged by tumors, large amounts of PSA are released into the bloodstream. However, PSA levels can also rise due to many other conditions not related to cancer, such as an enlarged, inflamed, or infected prostate. Conversely, PSA levels can be lowered in men taking certain medications, including finasteride and dutasteride, which are used to treat benign prostatic hyperplasia (BPH). Also, studies have shown that PSA levels gradually increase with age, even in the absence of prostate cancer or other conditions. This gradual increase is largely due to the natural enlargement of the prostate as men age.
PSA screening and prostate cancer
Because prostate cancer often develops slowly and may not exhibit symptoms at early stages, PSA testing is a valuable screening tool in detecting prostate cancer. When detected and treated in its early stages, the overall prognosis for prostate cancer is generally good, with a high likelihood of successful treatment and long-term survival. However, a high PSA level does not necessarily indicate cancer, as many men with elevated PSA levels do not have prostate cancer. On the other hand, some men diagnosed with prostate cancer may have PSA levels within the normal range. Thus, the PSA test is not a definitive screening tool for prostate cancer. If the PSA level is found to be elevated, the clinician may recommend a follow-up PSA test after a few weeks to confirm the initial finding and/or perform digital rectal examination (DRE) to check for abnormalities in the size, shape, or texture of the prostate. Alternatively, imaging tests like magnetic resonance imaging (MRI) and/or a prostate biopsy may be recommended without conducting additional tests. During the biopsy, multiple samples of prostate tissue are taken and examined under a microscope for the presence of cancer cells.
The need for more accurate diagnostic tests
Undoubtedly, PSA testing plays a central role in the early detection and management of prostate cancer, which has improved outcomes for many patients. However, the test has also been widely associated with overdiagnosis and overtreatment, sparking an ongoing
debate in the medical community about the potential risks and benefits of routine PSA screening. A 2022 systematic review and meta-analysis study published in BMC Medicine reported a pooled sensitivity of 0.93 (95% CI, 0.88–0.96) and a specificity of 0.20 (95% CI, 0.12–0.33) for the PSA test using a 4 ng/mL threshold in detecting prostate cancer in symptomatic patients. This indicates that while the test has good sensitivity, its low specificity leads to a high number of false positives, causing unnecessary anxiety and biopsies for individuals who do not actually have cancer. The need for more accurate diagnostic tests has driven extensive research into alternative or complementary tests to PSA. As a result, several novel approaches have emerged, some of which have demonstrated promising results in detecting prostate cancer, including a urine-based test that analyzes biochemical profiles. These tests not only aim to improve the sensitivity of early detection but also seek to reduce the number of false positives and unnecessary biopsies, providing patients with a more reliable and less invasive diagnostic experience. Furthermore, on-going efforts are being made to use these tests to identify aggressive forms of prostate cancer earlier, enabling more tailored and effective treatment strategies while avoiding unnecessary interventions for non-life-threatening prostate cancers.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.