The traditional approach to cancer classification
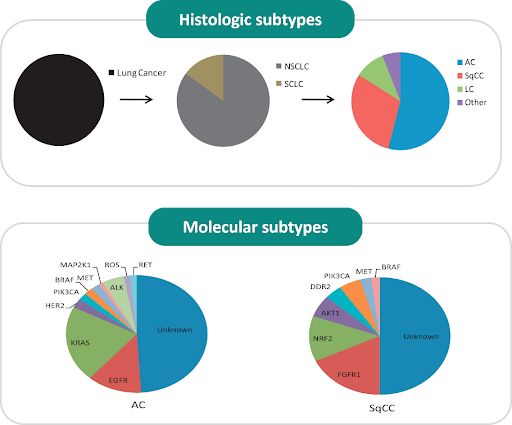
Cancers are traditionally classified based on their organ or tissue of origin, such as breast, lung, or liver cancer. This classification, where tumors within each cancer are subtyped using histology and staging systems, has long guided diagnosis, prognosis, and treatment decisions. This approach assumes that tumors originating in the same organ share similar biological behaviors and prognoses, leading to treatment strategies that were largely generalized for each cancer type. While this framework has been instrumental and remains the foundation of cancer care today, it does not fully capture the complexity of cancer biology. Mounting evidence has shown that tumors originating from the same organ can vary in terms of gene mutations, gene expression profiles, and treatment response. This is known as tumor heterogeneity. For example, breast cancers may have vastly different molecular profiles, such as HER2-positive and triple-negative, with each requiring a distinct therapeutic strategy. Conversely, tumors from different organs may share some common genetic alterations and exhibit similar response to the same treatment, even if their anatomical origins differ. This realization has prompted a clarion call to shift from organ- and histology-based classification to molecular classification.
The advent of molecular classification in precision oncology
Molecular classification refers to the stratification of cancers based on their unique molecular characteristics, rather than solely on their primary site of origin or histological characteristics. This approach identifies key mutations, gene expression profiles, and molecular pathways that drive tumor growth and influence therapeutic outcomes. For instance, breast cancers can now be molecularly classified into subtypes such as HER2-positive, hormone receptor positive, or triple-negative, each with distinct biological behaviors and therapeutic implications. Likewise, non-small cell lung cancer can also be subtyped based on the presence of mutations in genes such as EGFR, ALK, RET, or ROS1. The key advantage of molecular classification is that it enables more personalized approach to cancer care by matching therapies to the specific genetic makeup of each patient’s tumor, leading to better treatment outcomes, reduced adverse effects, and a more efficient use of the healthcare resources. By focusing on the molecular pathways rather than specific cancer types, this facilitates drug development and clinical trial designs that are more targeted and predictive of treatment response. One of the most significant breakthroughs in this area is the tumor-agnostic therapies – drugs that are approved for use across various cancer types based on shared genetic alterations. Pembrolizumab (Keytruda) became the first tumor-agnostic therapy when it was approved by the US FDA in May 2017 for the treatment of solid tumor patients with microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR), regardless of the tumor’s origin.
Next-generation sequencing accelerating the shift to molecular classification
Next-generation sequencing (NGS) technologies have been a pivotal component in shifting the focus from the anatomical location of a tumor to its molecular characteristics. NGS enables rapid and comprehensive analysis of tumor genomes, analyzing a wide range of genetic alterations such as point mutations, insertions and deletions, copy number variations, and gene fusions, within a single assay. Unlike earlier methods that were limited to analyzing one or several genes at a time, NGS can assess hundreds to thousands of genes simultaneously with high sensitivity and specificity, providing a more comprehensive view of the tumor’s molecular profile. This high-throughput capability has made it possible to uncover novel and rare mutations across cancers of different tissue origins, helping to develop and guide the use of targeted therapies and immunotherapies. In summary, instead of treating cancers based on their organ or tissue of origin, molecular classification allows for a more nuanced approach by tailoring therapies based on the molecular characteristics of the tumor. Looking ahead, the future of cancer classification lies in even deeper molecular insights using a multi-omics approach that integrates genomics, transcriptomics, proteomics, and epigenomics. Coupled with advances in artificial intelligence and machine learning to synthesize increasingly complex data, these innovations will pave the way for more effective and personalized cancer care.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.