The Role of Reference Materials in Next-Generation Sequencing
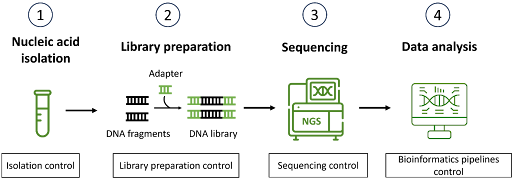
In next-generation sequencing (NGS), reference materials are crucial components in ensuring accuracy, consistency, and reliability of the entire NGS workflow. These are well-characterized genetic materials with known sequences or mutations at defined allelic frequencies or copy numbers and play an integral role in developing, optimizing, validating and monitoring NGS assays. One of the uses of reference materials is to assess the performance of the sequencing platforms. As there are various NGS platforms available in the market today, each with its own strengths and weaknesses, reference materials can help in benchmarking the different sequencing technologies. By using the same reference material across multiple sequencing platforms, different key metrics can be directly compared, such as read depth, coverage uniformity, error rates, performance in difficult-to-sequence regions, or detecting low-frequency variants. This allows the laboratory to select the best sequencing platform for their intended application while minimizing false positives and negatives. Reference materials are also frequently used during development of new NGS assays, enabling the assay performance to be optimized before being made available for clinical use. By using the same reference material, sequencing results can be directly compared with the known sequence to assess the entire NGS workflow, including library preparation, sequencing and bioinformatics pipelines, to determine the overall sequencing fidelity. While well-characterized, high-quality DNA or RNA reference materials are commonly used to reduce variability in molecular analyses, the use of reference materials in the form of formalin-fixed, paraffin-embedded (FFPE) curls or blocks offers the added advantage of evaluating the nucleic acid isolation step. This better mimics the challenges of isolating nucleic acids from formalin-fixed tissues, such as degradation and cross-linking, thereby more accurately representing clinical sample conditions. This is particularly important in clinical diagnostics, where sequencing specificity, sensitivity, and accuracy can influence patient diagnosis and treatment decisions. Reference materials also play an essential role in assessing assay repeatability (variation within same run) and reproducibility (variation between different runs). When conducting multiple sequencing runs or comparing results from different instruments and laboratories, using the same reference material allows for consistent comparison. This is important in harmonizing sequencing results between laboratories, especially in multi-center clinical trials or national testing programs. Reference materials are also used in routine laboratory operations as part of the internal quality control measures to identify potential technical issues that could affect the quality of the results and ensure robust assay performance. In clinical diagnostics, adherence to regulatory standards such as those set by the Clinical Laboratory Improvement Amendments (CLIA) or the College of American Pathologists (CAP) often requires the use of validated reference materials to demonstrate that the testing process consistently meets stringent performance criteria. These standards help ensure that laboratories can provide timely and accurate results with a high degree of confidence. In conclusion, reference materials have become an invaluable resource in NGS testing, enabling robust assay development, thorough validation, and performance consistency verification. As NGS technologies continue to evolve, the role of reference materials becomes even more pivotal. They not only maintain the integrity of the results, but also support the standardization of protocols across different laboratories and platforms. Ultimately, the use of reference materials helps ensure the accuracy, repeatability, and reproducibility of sequencing results, while maintaining the high standards in clinical diagnostics.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.