Understanding Surrogate Endpoints in Cancer Research
The development of new cancer therapies is a complex, time-consuming, and costly process. Clinical trials, which are essential for evaluating the efficacy and safety of new treatments, often take years to deliver meaningful results, especially when survival outcomes are the primary endpoint. To expedite this process and provide earlier insights into the treatment’s potential benefits, surrogate endpoints were introduced as substitutes for direct clinical outcomes, such as overall survival (OS) or quality of life, in clinical trials.
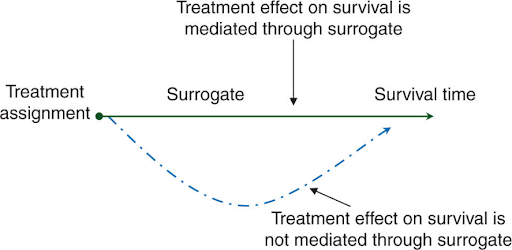
Surrogate endpoints in cancer trials
One of the most commonly used surrogate endpoints is progression-free survival (PFS), which refers to the length of time during and after treatment that a patient lives without the cancer worsening. While not a direct measure of OS, PFS can provide an early indication of how well a treatment is working, potentially speeding up the decision-making process in clinical trials. Another widely used surrogate endpoint is overall response rate (ORR), which measures the percentage of patients whose tumors shrink or disappear following treatment. ORR is especially useful for evaluating therapies in cancers that are challenging to treat or are at advanced stages, where waiting for OS data could take too long. While ORR may not guarantee survival benefit, it can serve as a valuable early marker of treatment efficacy. In some cases, pathological complete response (pCR) has also been used as a surrogate endpoint. Studies have shown that achieving pCR, defined as the lack of residual invasive cancer in resected tissue after neoadjuvant therapy, is often associated with better long-term survival outcomes. In recent years, minimal residual disease (MRD) has gained attention as surrogate endpoint, particularly in hematologic malignancies. MRD refers to the small number of cancer cells that remain below the detection threshold of conventional tests after treatment, but can be detected using highly sensitive flow cytometric or molecular assays like next-generation sequencing (NGS), shortly after therapy. Studies have shown a strong correlation between MRD negativity and improved clinical outcomes across multiple malignancies. Furthermore, MRD status can be used to predict disease relapse and guide treatment adjustments, therapy intensification or de-escalation during a clinical trial.
The advantages and challenges of surrogate endpoints
The attractiveness of surrogate endpoints lies in their ability to expedite drug development and approval by offering earlier indications of efficacy, thus reducing both the duration and cost of clinical trials. In therapeutic development, waiting for OS outcomes can take many years, particularly for cancers with slow progression. By providing earlier signs of clinical benefits, surrogate endpoints enable the accelerated approval of potentially life-saving therapies, allowing patients to access innovative treatments sooner. This is especially beneficial to patients with advanced or aggressive cancers, where rapid initiation of treatment is crucial for improving outcomes. Although surrogate endpoints can provide valuable insights, they are not without drawbacks. Not all of them consistently correlate with actual clinical outcomes, and some treatments that appear to improve surrogate measures may not translate into long-term survival benefits. For instance, certain therapies may shrink tumors rapidly but fail to extend survival, or their toxicities may severely compromise patients’ quality of life despite demonstrating good efficacy, ultimately causing more harm than good. Additionally, the association between these surrogate endpoints and OS can vary between cancer types and clinical trials, even if a strong link is found within a single study. The variations could be due to factors like differences in patient populations, the complexity of cancer biology, tumor heterogeneity, trial design, or the influence of other factors beyond the initial response to therapy. These discrepancies have raised concerns about the reliability of surrogate endpoints and the potential risks of approving therapies that may not provide meaningful long-term benefits.
Enhancing the reliability of surrogate endpoints
Undoubtedly, surrogate endpoints play a pivotal role in speeding up the development and approval of cancer therapies, offering a more efficient pathway to bringing new treatments to patients. However, they must be carefully validated through rigorous scientific studies and comprehensive data analysis. Researchers must ensure that these markers can reliably predict actual clinical outcomes in the context of the specific cancer type being treated, and the duration of response is adequately assessed to minimize the risk of transient responses that do not yield long-term benefit. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), have established guidelines for the use of surrogate endpoints, ensuring that there is sufficient evidence linking these markers to long-term survival or other meaningful clinical benefits. Importantly, post-marketing studies and on-going surveillance must be carried out after approval to confirm the continued effectiveness and safety of treatments for patients.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.