Introduction
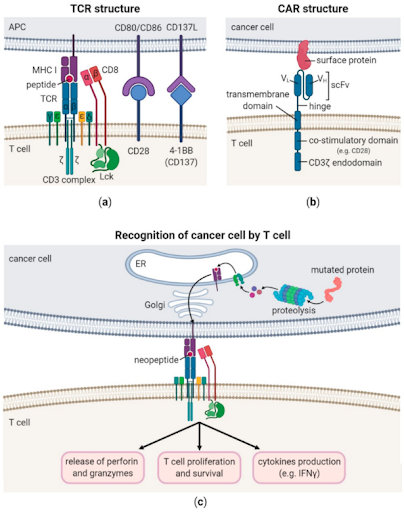
The T-cell receptor (TCR) is a molecule found on the surface of T cells, responsible for recognizing specific antigens presented by major histocompatibility complex (MHC) molecules. When TCR binds to the antigen-MHC complex, it initiates a cascade of intracellular signaling that activates the T cell and triggers an immune response.
TCRs regulate immune response
The TCR is a heterodimeric transmembrane protein that belongs to the immunoglobulin superfamily. Around 90-95% of TCRs consist of alpha (α) and beta (β) chains, known as αβ T cells, while the remaining TCRs are composed of gamma (γ) and delta (δ) chains, called γδ T cells. Each chain contains a variable region for antigen recognition, and a constant region for maintaining structural integrity of the receptor. The TCR’s variable regions are unique to each T cell, allowing the immune system to recognize diverse antigens. Activation of T cells begins when TCRs interact with antigens presented by MHC molecules, leading to its proliferation and differentiation. When CD8+ cytotoxic T cells bind to antigens presented by MHC class I molecules, which are present on nearly all nucleated cells, CD8+ cytotoxic T cells release perforins and granzymes to directly induce cell death in targeted cells. In contrast, CD4+ T helper cells interact with antigens presented by MHC class II molecules, which are mainly found on antigen-presenting cells such as dendritic cells and macrophages. Upon activation, CD4+ helper T cells secrete cytokines that are crucial for coordinating the broader immune response, including promoting activation of CD8+ cytotoxic T cells and stimulating B cells to produce antibodies. TCR signaling is tightly regulated to prevent excessive immune reactions, such as autoimmunity. Besides regulatory T cells (Tregs), a subset of CD4+ T cells, which help to control immune response by suppressing overactivation, inhibitory signals like PD-L1 provide negative feedback to limit immune response and avoid tissue damage.
Challenges of CAR T-cell therapy in solid tumor treatment
Continuous research in cancer treatments has led to innovative personalized approaches such as adoptive cell therapy, whereby isolated immune cells are enhanced and/or engineered in the laboratory before being infused into cancer patients to boost the immune system in fighting against cancer. An example of adoptive cell therapy is chimeric antigen receptor (CAR) T-cell therapy. In the laboratory, the collected T cells are genetically modified to express synthetic CAR on their surface, designed to recognize antigens commonly found on surface of cancer cells (for example, CD19 on lymphoma cells). These modifications, which enable T cells to better recognize and attack cancer cells, have led to breakthroughs in the treatment of certain cancers. Although several CAR T-cell therapies have been approved by the U.S. Food and Drug Administration (FDA) for hematologic malignancies, their effectiveness in solid tumors remain limited due to immunosuppressive tumor microenvironment, lack of tumor-specific surface antigens, and dense tumor structures that hinder CAR T-cell infiltration.
Advantages of TCR therapy in solid tumor treatment
In recent years, TCR therapy has emerged as a promising therapeutic approach for the treatment of solid tumors, owing to several key advantages. In contrast to CAR T-cells that only recognize surface antigens on the cancer cells, TCRs can recognize both surface and intracellular antigens, broadening the pool of tumor associated antigens as potential targets and enabling more cancer types to benefit from this approach. Additionally, since TCRs can interact with MHC-presented antigens, it is thought that this can facilitate deeper tumor infiltration and increased activity within the tumor tissue, improving efficacy of TCR therapy in the microenvironment of solid tumors. Moreover, studies have shown that TCRs demonstrated greater sensitivity and mediate lower levels of cytokine release, when compared to CAR T-cells. The key drawback for TCR therapy, in comparison to CAR T-cell therapy, is its reliance on antigen recognition through MHC molecules. Consequently, the effectiveness of TCR therapy depends on the patient’s unique human leukocyte antigen (HLA) profile.
First TCR therapy approved by the U.S. FDA for cancer
Afamitresgene autoleucel (Tecelra, Adaptimmune, LLC) became the first TCR therapy to be approved by the U.S. FDA for cancer on August 02nd, 2024, after it demonstrated 43% overall response rate and median duration of six months among the 44 patients who received Tecelra in a clinical trial. Tecelra, which takes around 6 weeks to manufacture and is administered as a single intravenous dose, is indicated for the treatment of adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are HLA antigen(s) A02:01P, -A02:02P, -A02:03P, or -A02:06P positive, and whose tumor expresses the melanoma-associated antigen A4 (MAGE-A4) antigen.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.