Introduction
Next-generation sequencing (NGS), a high-throughput method that rapidly sequences DNA or RNA for comprehensive analysis of mutations driving cancer development and progression, has become an essential tool across the cancer care continuum. The integration of this advanced technology into every phase of cancer care has become the cornerstone of precision oncology, empowering clinicians to make data-driven decisions that optimize prevention and treatment strategies.
Risk assessment
Through an in-depth analysis of specific gene mutations that contribute to cancer development such as BRCA and APC genes, NGS allows for the early identification of individuals at elevated risk of developing certain cancers such as breast, ovarian, or colorectal cancer. This information facilitates risk stratification, enabling prevention and early detection strategies tailored to the individual’s unique genetic profile, including enhanced surveillance, prophylactic surgeries, or lifestyle modifications. Additionally, NGS aids in identifying inherited mutations that may be passed on to future generations and at-risk family members, thus contributing to family-wide prevention strategies.
Early detection
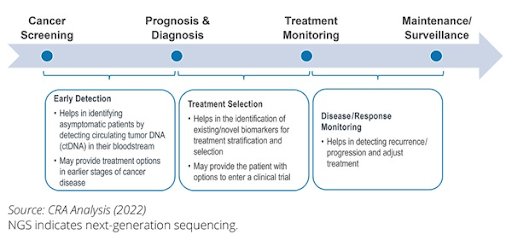
NGS enables detection of cancer-associated biomarkers in asymptomatic individuals with high sensitivity and specificity. By analyzing the genetic profiles of cell-free DNA (cfDNA) or circulating tumor DNA (ctDNA) from liquid biopsies, NGS can identify subtle genetic changes that indicate the presence of malignancy, sometimes even in the absence of clinical symptoms. Early detection not only improves treatment outcomes, but also improves quality of life and reduces treatment burdens. Thus, early cancer detection is particularly transformative for cancers without established screening guidelines such as pancreatic and liver cancer.
Diagnosis and prognosis
NGS plays a pivotal role in cancer diagnosis and prognosis by providing detailed insights into the genetic makeup of tumors, which helps to identify cancer subtypes and mutations. Molecular classification aids clinicians to diagnose cancer and assess prognosis more accurately by identifying biomarkers that correlate with tumor behavior, aggressiveness, recurrence potential, and response to treatment. This approach enhances clinical decision-making, improves prognostication, and allows treatment strategies tailored to the unique molecular characteristics of the tumor.
Treatment selection
Targeted therapies and immunotherapies have significantly improved survival rates and quality of life by shifting treatment strategies from a one-size-fits-all approach to personalized approach. The expanding list of approved therapies underscores the importance of tumor genomic profiling, with NGS emerging to the forefront to provide detailed molecular insights that guide clinicians to select the most effective therapies for each patient. This personalized approach not only enhances treatment efficacy but also minimizes unnecessary side effects by avoiding ineffective therapies. Furthermore, NGS allows matching of patients to clinical trials, especially basket trials that group patients based on shared genetic alterations rather than tumor location.
Monitor treatment response
Studies have shown that blood-based ctDNA level correlates with tumor burden and treatment response in various cancers, indicating its utility in monitoring treatment response. Through longitudinal monitoring via liquid biopsy, NGS can be used to provide insights into treatment efficacy and guide treatment escalation or de-escalation. Moreover, NGS can also monitor tumor evolution and track the emergence of resistance mutations that can develop during the treatment course, allowing clinicians to switch to alternative therapy promptly, ensuring more effective management of the cancer.
Pharmacogenomics
Genetic variations can greatly influence how medications are processed in the body due to inherited mutations that can affect both pharmacokinetics and pharmacodynamics. NGS helps to optimize cancer treatment by identifying genetic variations that influence drug metabolism, efficacy, and toxicity, guiding dose adjustments or therapy changes. This personalized approach ensures that not only the right drug but also the right dose is administered based on the patient’s genetic profile, enhancing treatment efficacy and preventing adverse drug reactions.
Monitor recurrence
Patients who have successfully undergone cancer treatment are usually monitored at regular intervals for signs of recurrence. Post-treatment surveillance is another area where NGS is proving to be transformative. With its high sensitivity and ability to analyze genetic alterations at a much deeper level than traditional methods, NGS can track minimal residual disease (MRD) by monitoring ctDNA in the blood, identifying recurrences before clinical or radiologic signs appear. Early detection enables intervention before disease progression, thereby increasing the likelihood of positive outcomes. In summary, NGS has emerged as an indispensable tool in oncology, revolutionizing cancer care by enabling personalized, data-driven management strategies. Its utility spans the entire cancer care continuum, from risk assessment and early detection to treatment planning and disease surveillance. Ultimately, NGS empowers clinicians to make informed decisions that significantly improve patient outcomes by optimizing therapies based on the unique molecular characteristics of both the patient and the tumor.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.