Introduction
Comprehensive genomic profiling (CGP) is an advanced molecular testing approach that simultaneously analyzes a broad panel of genes in cancer cells to identify various genomic alterations such as single nucleotide variants (SNVs), insertions and deletions (indels), copy number alterations (CNAs), and rearrangements. Unlike traditional approaches that test for a limited number of mutations in a single gene (or sometimes, in several genes), CGP uses next-generation sequencing (NGS) technology to examine hundreds to thousands of genes for multiple classes of mutations, including genomic signatures such as tumor mutational burden (TMB) and microsatellite instability (MSI) status in a single assay. Thus, CGP is being increasingly adopted in cancer diagnostics as it allows for a deeper understanding of a tumor’s genetic landscape, providing crucial insights to guide cancer diagnosis, treatment and prognosis. Although CGP is traditionally performed using tissue biopsy, advancements in technology have enabled liquid biopsy to be used as well, with each sample type offering its own advantages, limitations, and considerations.
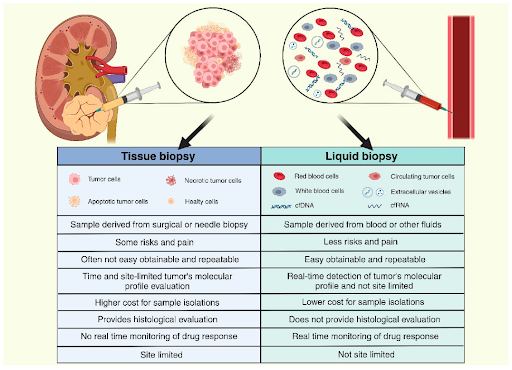
Tissue biopsy remains the gold standard in cancer diagnostics
Tissue biopsy has been a cornerstone in cancer diagnostics, particularly in solid tumors where tissue samples are readily accessible. Hence, it is still being routinely used in clinical settings to assess tumor characteristics and guide treatment decisions. Tissue samples provide direct examination of the tumor, offering accurate and detailed genomic information specific to the cancer cells themselves. Since the sample is taken directly from the tumor, it typically contains higher concentrations of genomic material, making it more likely to detect rare or low-abundance mutations. However, tissue biopsy requires invasive procedures such as surgery that carries certain risks, and some patients may be medically unfit to undergo such procedures. Tissue sampling can also be challenging if the tumor is too small or located in a difficult to assess area. In some cases, the amount of tissue available from a biopsy may not be sufficient for analysis. Because cancer is a heterogeneous disease, sampling at a single biopsy site might not fully capture the tumor’s heterogeneity, which could lead to incomplete understanding of the mutations driving the cancer. Lastly, repeated biopsies are not practical due to the invasive nature of the procedure, making tissue biopsy unsuitable for monitoring changes such as treatment response or detecting recurrence.
Liquid biopsy - bridging the gap in cancer diagnostics
Liquid biopsy, which uses samples from bodily fluids such as blood, offers a less invasive and more accessible option to tissue biopsy. Studies have shown that tumors release small fragments of DNA known as circulating tumor DNA (ctDNA) into the bloodstream, which can be analyzed by NGS. Liquid biopsy is particularly useful for patients who are medically unfit for invasive procedures, or in cases where the tumor is not easily accessible via needle or surgical procedures. Because liquid biopsy allows repeated samplings over time and offers shorter turnaround times than tissue biopsy, it can be used to monitor treatment response, identify emergence of resistance mutations, and detect early signs of relapse before symptoms manifest or tumors are visible through imaging methods. Another potential benefit is that ctDNA is more likely to represent a broader mix of subclones from both primary tumor and metastases (if any), compared to a single biopsy site. This can help to address the intra- and intertumoral heterogeneity, providing a more comprehensive analysis. While liquid biopsy is useful, it is not without drawbacks. One of the primary limitations is the reduced sensitivity compared to tissue biopsy, particularly in early-stage cancers when tumors are small or have a low tumor burden, causing ctDNA to be present in very low concentrations in the bloodstream. This can potentially lead to false negatives; whereby liquid biopsy might fail to detect the presence of mutations that could be critical to treatment decisions. ctDNA levels also vary according to cancer type. Tumors located in the central nervous system release very low levels of ctDNA into the bloodstream, likely due to the blood-brain barrier. Furthermore, detecting ctDNA can also be challenging in certain other cancers, such as thyroid and kidney cancers. In contrast, ctDNA can be more easily detected in cancers such as breast, lung and colorectal cancers, especially at advanced stages. Moreover, clonal hematopoiesis of indeterminate potential (CHiP), which are age-related mutations in blood cells and unrelated to cancer, can lead to false positive results in liquid biopsy.
Liquid biopsy as an alternative or complement to tissue biopsy
Despite the growing body of evidence demonstrating the clinical utility of liquid biopsy, it does not replace tissue-based diagnoses. Since tumor histology, grading and staging cannot be assessed through liquid biopsy, tissue biopsy remains essential for accurate cancer diagnosis and treatment. Aside from being commonly used for monitoring during or after treatment, liquid biopsy may also be employed when faster results will be clinically important, or when tissue biopsies are not possible or inadequate, according to clinical practice guidelines. In some cases, both tissue and liquid biopsies may even be performed simultaneously for a more comprehensive view of the tumor’s genetic profile to improve treatment outcomes and clinical trial eligibility. Although studies have demonstrated that this concurrent approach can mitigate the shortcomings of each method and improve the detection of actionable mutations, its high costs and lack of infrastructure to integrate both datasets in resource-limited settings hinder widespread adoption in practice. In summary, both tissue and liquid biopsies offer valuable insights, with the choice between them (or both) depending on clinical context, including patient’s condition, the type of cancer, and goal of the test.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.