Introduction
Liquid biopsy has revolutionized cancer care by enabling the non-invasive detection and analysis of tumor-derived biomarkers such as circulating tumor DNA (ctDNA) from body fluids such as blood, saliva, and urine. Next-generation sequencing (NGS), which allows for the analysis of low-frequency and genome-wide alterations in ctDNA with incredible sensitivity and precision, has emerged as a powerful tool for minimal residual disease (MRD) detection. MRD refers to the small number of cancer cells that may remain in the patient’s body after treatment, often undetectable by standard imaging or clinical examination. However, these residual cells can eventually lead to a relapse, making it crucial to monitor these cells post-treatment to assess treatment efficacy and identify patients at higher risk of relapse. By analyzing ctDNA, which serves as a marker of MRD, minute traces of cancer cells that would otherwise be missed can be detected early. This early detection capability is transformative, as it allows for more tailored and timely interventions – whether to adjust treatment plans or consider additional therapies to prevent recurrence. In general, there are two approaches to track the presence of any residual disease after treatment via liquid biopsy: tumor-informed and tumor-agnostic assays.
Tumor-informed assays
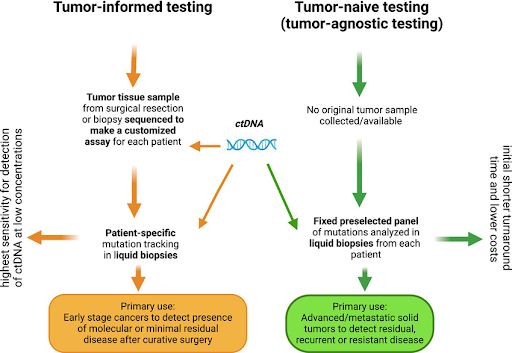
Tumor-informed assays are designed to analyze the genetic alterations specific to a patient’s tumor. In this approach, initial tumor profiling is first performed on the patient’s tissue biopsy at diagnosis to identify unique driver mutations (single nucleotide variants, structural variants, insertions and deletions) that are specific to that tumor. Typically, the initial tumor profiling is done using either whole-genome sequencing (WGS), whole-exome sequencing (WES) or comprehensive genomic profiling (CGP) to increase the likelihood of identifying relevant mutations. This information is then used to create a custom assay to detect MRD in the patient by tracking the same mutations over time. Because the assay is tailored to the tumor’s unique molecular signature, unrelated mutations such as mutations that arise from clonal hematopoiesis of indeterminate potential can be filtered out, minimizing the chances of false-positive and false-negative results. However, this approach is not without drawbacks. Tumor-informed assays require prior tumor profiling, but tissue biopsy may not always be available. This may be due to various reasons such as a patient may be medically unfit for the invasive procedure, the tumor may not be easily accessible, or the biopsy may have insufficient or low-quality material. Additionally, the assay is limited to known mutations identified from initial tumor profiling. Hence, rare or novel mutations due to tumor heterogeneity and clonal evolution may be missed, causing MRD to go undetected if these mutations are not included in the custom panel. It is also worth noting that the initial development of tumor-specific assays typically requires about four weeks, which could affect the patient’s prognosis.
Tumor-agnostic assays
Tumor-agnostic assays, on the other hand, do not rely on specific genetic alterations identified from the patient’s tumor. Therefore, this approach offers a valuable alternative when tissue biopsies are unavailable or in cases of cancers of unknown primary. Since tumor-agnostic assays use pre-designed, off-the-shelf panels that focus on a wide range of mutations commonly found across different cancer types, they are ready for immediate use compared to tumor-informed assays that need to undergo custom development for each case. Hence, tumor-agnostics assays can be more cost-effective and timesaving. The goal of tumor-agnostic assays is to detect low-frequency mutations (or other molecular markers) that are indicative of residual cancer cells, regardless of the specific cancer type or mutation. But because the same generic panel is utilized for all patients, only a subset of mutations in the panel are expected to be present in each patient’s residual disease, potentially reducing the sensitivity of the assay. While tumor-informed assays are thought to offer better performance, many tumor-agnostic assays now have specific features designed to enhance sensitivity and specificity, including robust bioinformatic tools to distinguish MRD signals from background noise within a large amount of complex genomic data. In addition, tumor-agnostics assays can also address some of the limitations of tumor-informed assays, such as tumor heterogeneity and the emergence of new mutations. With both approaches having their own strengths and limitations, the choice between tumor-informed and tumor-agnostic assays largely depends on the availability of tissue biopsy, clinical context and testing goals. Regardless of the choice, NGS-powered liquid biopsy offers a non-invasive approach to monitor MRD status in real-time with high sensitivity and precision. This has a profound impact in improving long-term outcomes for cancer patients, as early detection of MRD can significantly influence clinical decision-making, enabling more personalized and effective cancer management strategies.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.