Introduction
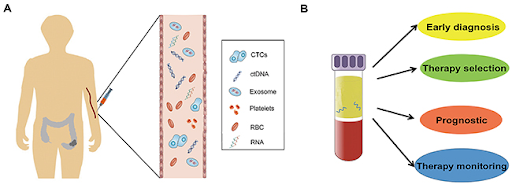
Liquid biopsy is a minimally invasive or non-invasive approach that analyzes various biomarkers in body fluids such as blood, urine, or saliva, to detect and monitor diseases, including cancer. It has gained widespread attention in cancer management in recent years due to its potential for early screening, disease monitoring, identification of driver mutations, and assessment of treatment responses. While liquid biopsy is usually associated with circulating tumor DNA (ctDNA), there are also other biomarkers that can be detected via liquid biopsy.
Circulating tumor DNA (ctDNA)
One of the most commonly studied biomarkers in liquid biopsy, ctDNA refers to the small fragments of DNA that are shed from cancer cells into the bloodstream. ctDNA is a subtype of cell-free DNA (cfDNA), a broader term used to describe all DNA fragments found in the bloodstream, which can originate from various sources, including healthy cells, tumor cells, or even microbes. Since ctDNA carries tumor-specific genetic mutations, copy number alterations, and epigenetic modifications, this makes ctDNA particularly useful for identifying cancer-related mutations, monitoring tumor burden, detecting minimal residual disease, and assessing therapeutic responses. The sensitivity of ctDNA can be quite high, especially in advanced cancers with high mutation rates, such as lung and colorectal cancers. On the other hand, ctDNA may not be readily detectable in early-stage cancers or in cases where the tumor sheds little DNA into the bloodstream.
Circulating tumor cells (CTCs)
Unlike ctDNA, which is derived from dead or dying tumor cells, CTCs are living tumor cells that detach from the tumor and enter the bloodstream. These cells are important because they can potentially form metastases and are often found in cancers that have spread beyond their primary site. Thus, the detection of CTCs can provide valuable information about the metastatic potential of the cancer and help with early detection of relapse. Additionally, CTCs also retain transcriptome profiles, enabling comprehensive gene expression analysis. However, their isolation and analysis can be technically challenging due to their low concentration in the blood. Nevertheless, advancements in microfluid technologies have improved the detection sensitivity of CTCs, allowing for more accurate profiling of tumor characteristics.
MicroRNAs (miRNAs)
miRNAs are small non-coding RNA molecules that regulate gene expression by binding to target mRNAs, inhibiting their translation. Tumor cells are known to release miRNAs into the bloodstream, and changes in the expression profiles of these miRNAs can reflect tumor-specific behavior. The advantages of miRNAs include their stability in circulation, being less prone to degradation, and involvement in regulating multiple cellular processes, such as proliferation, apoptosis, and metastasis. These characteristics make them a promising tool for cancer diagnosis, prognosis, and treatment monitoring. However, their expression profiles can vary depending on tumor type and stage, and being less studied than ctDNA, a larger validation cohort is needed to establish miRNAs as a reliable biomarker in clinical practice.
Circulating tumor RNA (ctRNA)
In contrast to ctDNA, ctRNA refers to RNA fragments that are released by tumor cells into the bloodstream, which includes both coding (mRNA) and non-coding RNA (like miRNA). ctRNA (particularly mRNA) provides a real-time view of which genes are being actively transcribed, which is important in understanding tumor biology, disease progression, and treatment resistance. miRNA, which is part of ctRNA, can be used to profile tumor-specific regulatory mechanisms. A drawback of ctRNA is that it is typically more prone to degradation by ribonucleases in the bloodstream, making it less stable than ctDNA.
Exosomes
Exosomes are small vesicles (30-150 nm in diameter) that are secreted by many different cell types, including tumor cells. They contain various molecular cargo, such as proteins, lipids, RNA, and DNA, which reflect the state of their parent cells. Exosomes are considered to be a promising biomarker because they provide a snapshot of the molecular changes occurring within a tumor, which lies with their ability to carry a wide range of molecular information, including mRNA, non-coding RNA, and even ctDNA. This makes them useful for identifying specific mutations, gene expression patterns, and other molecular changes that can be used for diagnosis, monitoring treatment response, and identifying resistance mechanisms. However, isolating exosomes is considered challenging due to their small size, biological sample complexity, inherent heterogeneity, and tendency to co-segregate with other similar structures.
Proteins
Proteins are another biomarker that can be detected in liquid biopsy, often through techniques such as mass spectrometry or immunoassay. Tumor markers or cancer antigens (such as prostate-specific antigen (PSA) for prostate cancer and cancer antigen-125 (CA-125) for ovarian cancer) are widely used to detect tumor presence and progression globally. The challenge with these first-generation protein-based biomarkers is their potential for high variability due to factors like assay sensitivity, and the influence of non-tumor-related conditions. Additionally, they may not always be cancer-specific and can be influenced by other diseases or even normal biological processes.
Tumor-educated platelets (TEPs)
TEPs are platelets that have been modified by exposure to tumor cells, as part of the body’s response to cancer. When cancer cells are growing and spreading, they release various substances, such as growth factors, metabolites, and cytokines into the bloodstream. Platelets, which are small cell fragments involved in blood clotting, can absorb some of these signals, essentially educating them to behave differently from normal platelets. This phenomenon has sparked interest in using TEPs for cancer diagnostics, as their molecular signatures may reflect the presence and type of cancer, potentially allowing for early detection or monitoring of tumor progression. However, research is still in its early stages, and there are challenges in distinguishing TEPs from normal platelets. Moreover, the analysis of TEPs require sophisticated techniques to isolate and analyze the platelets due to their small size and the complexity of the tumor signals that they carry.
Tumor-associated autoantibodies (TAAs)
TAAs are antibodies that the immune system produces in response to tumor antigens. These autoantibodies are often detected in cancer patients, and their presence can be used as an indicator of early-stage cancer, or as a marker for cancer progression. Unlike biomarkers that directly reflect tumor DNA or cells, TAAs provide insight into the immune response instead. Some studies have shown that TAAs can be detected in the bloodstream even before clinical symptoms appear, enabling early diagnosis of cancers such as lung cancer in high-risk individuals. One major challenge with TAAs is the complexity of the immune response, which can be highly individualized. Different cancer types may produce distinct autoantibodies, and not all patients will have detectable autoantibodies, making it difficult to rely on TAAs alone for a universal diagnostic test. In summary, liquid biopsy is evolving rapidly and has the potential to provide crucial insights into tumor biology, facilitate early cancer detection, identify tumor mutations, monitor disease progression, assess treatment efficacy, and track resistance mechanisms. Each type of biomarker has its own strengths and limitations, and their combined use may enable more comprehensive diagnostic and prognostic information to guide treatment decisions in clinical practice.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.