An Overview of Immunotherapy Approaches
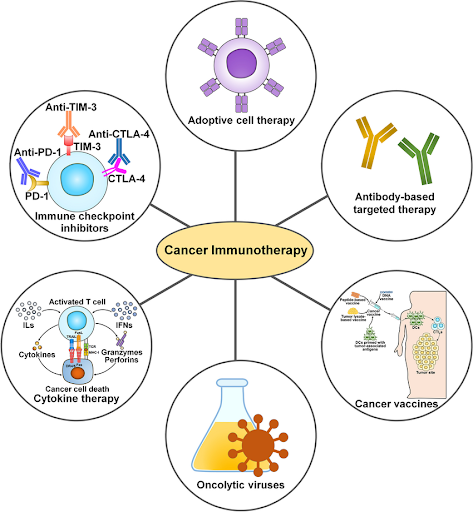
Immunotherapy represents a groundbreaking approach in the treatment of cancer, empowering the body’s own immune system to recognize and destroy malignant cells. Unlike conventional therapies which act directly on cancer cells, immunotherapy works by boosting or restoring the immune system’s ability to fight cancer more effectively. The immune system is naturally equipped to detect and eliminate abnormal cells, but cancer can evolve mechanisms to evade immune detection, such as by suppressing immune response or disguising itself as normal cells. Immunotherapy aims to overcome these challenges through various innovative strategies, which can be broadly categorized into five major types: immunomodulators, targeted antibodies, adoptive cell therapy, cancer vaccines, and oncolytic virus therapy.
Immunomodulators, which include immune checkpoint inhibitors, cytokines, and agonists, are a class of immunotherapy agents that regulate the activity of the immune system to enhance its ability to recognize and eliminate cancer cells. Immune checkpoint inhibitors work by blocking checkpoint proteins that normally act as “brakes” to prevent the immune system from attacking healthy, normal cells, but which cancer cells often exploit to avoid immune detection. For example, pembrolizumab (Keytruda), atezolizumab (Tecentriq), and ipilimumab (Yervoy) block checkpoint proteins like PD-1, PD-L1, and CTLA-4, enabling T-cells to better recognize and attack cancer cells. Cytokines are messenger molecules that play an important role in facilitating communication between immune cells to coordinate a targeted response against antigens. Interferon-alpha 2 (IFN-α2) was the first cytokine approved by the U.S. Food and Drug Administration (FDA) when it gained approval for the treatment of hairy cell leukemia in 1986. Agonists work by mimicking the natural ligands or bind to specific receptors on immune cells to activate pathways that promote adaptive immune responses. For example, the nogapendekin alfa inbakicept-pmln (Anktiva), an interleukin-15 (IL-15) receptor agonist that activates natural killer cells, T-cells, and memory T-cells, was approved by the U.S. FDA in 2024, in combination with Bacillus Calmette-Guérin (BCG), for the treatment of BCG-unresponsive non-muscle invasive bladder cancer (NMIBC).
Targeted antibodies are engineered proteins designed to specifically recognize and bind to target antigens present on the surface of cancer cells to either directly kill cancer cells, deliver anti-cancer drugs, or flag them for destruction by immune cells. Monoclonal antibodies (mAbs) are monospecific antibodies consisting of variable regions that bind to a specific antigen on the cancer cell and a constant region that interacts with the immune system to facilitate cell destruction. In 1997, rituximab (Rituxan), which targets CD20 antigen on B cells, became the first mAb approved by the U.S. FDA for the treatment of cancer. Unlike regular mAbs, antibody-drug conjugates (ADCs) combine a mAb with a cytotoxic payload via a chemical linker. The antibody directs the ADC to the cancer cell by binding to a specific antigen. After binding, the ADC is internalized, and the cytotoxic drug is released inside the cell, leading to rapid cancer cell death. The first U.S. FDA-approved ADC was gemtuzumab ozogamicin (Mylotarg), which was approved in 2000 for the treatment of acute myeloid leukemia (AML). While mAbs can only bind to one specific antigen, bispecific
antibodies work by simultaneously binding to two different targets, typically a cancer cell and an immune cell like a T-cell, to bring them into close proximity, activating the T-cell to destroy the cancer cells. In 2014, blinatumomab (Blincyto) was approved by the U.S. FDA as the first bispecific antibody to treat acute lymphoblastic leukemia (ALL), targeting both the CD19 antigen on B-cells and the CD3 antigen on T-cells.
Adoptive cell therapy, also known as cellular immunotherapy, is a form of immunotherapy that involves transferring immune cells into a cancer patient’s body to fight the disease. These cells are collected from the patient (or a healthy donor), expanded exponentially in a laboratory, and sometimes genetically engineered to enhance their cancer-fighting capabilities before being infused into the patient to boost the immune system’s ability to combat cancer. One of the most prominent forms of adoptive cell therapy is the chimeric antigen receptor T-cell therapy (CAR T-cell therapy), in which a patient’s T-cells are genetically engineered to express synthetic a receptor known as CAR to better recognize and target cancer cells. The first CAR T-cell therapy, tisagenlecleucel (Kymriah), received the U.S. FDA approval in 2017 for the treatment of ALL. Other innovative approaches include tumor-infiltrating lymphocyte (TIL) and T-cell receptor (TCR) therapies. TIL therapy involves isolating T-cells that have already infiltrated the patient’s tumor, expanding them in the laboratory, and reinfusing them back into the patient. On the other hand, TCR therapy involves genetically modifying T-cells isolated from the patient in the laboratory to express specific receptors capable of recognizing tumor-associated antigens (TAAs) before being reinfused back into the patient. Unlike traditional CAR T-cells that only recognize surface antigens, TCRs can recognize both surface and intracellular antigens. To date, only one TIL therapy, lifileucel (Amtagvi) approved in 2024 for melanoma, and one TCR therapy, afamitresgene autoleucel (Tecelra) approved in 2024 for synovial sarcoma, have been approved by the U.S. FDA for cancer treatment. Recently, adoptive cell therapy has expanded to explore other immune cells, with natural killer (NK) cells emerging as a promising candidate, particularly due to their potential to be developed as an “off-the-shelf” therapy. While no NK cells-based therapies have been approved by the U.S. FDA yet, several investigational products in various stages of clinical development have shown high efficacy and good safety profiles.
Cancer vaccines represent one of the most exciting advancements in oncology. There are two main types: preventive (prophylactic) vaccines and therapeutic vaccines. Preventive vaccines are given to healthy individuals to prevent infections known to cause certain types of cancer, while therapeutic vaccines focus on treating existing cancer by boosting the body’s immune response. One of the most well-known preventive cancer vaccines is the human papillomavirus (HPV) vaccine, which protects against many strains of HPV responsible for causing cervical, anal, penile, and other cancers. Studies have shown that the HPV vaccine has led to dramatic reduction in HPV-related cancers, especially cervical cancer. Besides the HPV vaccine, the hepatitis B vaccine which protects against hepatitis B virus (HBV), a major risk factor for liver cancer, is also available. On the therapeutic side, two vaccines have been approved by the U.S. FDA for the treatment of cancer: BCG was approved for patients with NMIBC in 1990 and Sipuleucel-T (Provenge) was approved for patients with prostate cancer in 2010. Researchers are also exploring new approaches,
including messenger RNA (mRNA) vaccines, which gained prominence during the Covid-19 pandemic. mRNA cancer vaccines work by encoding TAAs, which are then translated into proteins in the body to stimulate an immune response against the target cancer. The development of mRNA cancer vaccines is a rapidly advancing field, with over 100 clinical trials ongoing worldwide. In 2023, mRNA-4157 (V940), developed by Moderna and Merck, became the first mRNA cancer vaccine to progress to phase 3 clinical trials. This was initiated following encouraging results from phase 2 trials, where the vaccine in combination with pembrolizumab demonstrated promising results when used as adjuvant treatment in patients with resected high-risk melanoma.
Oncolytic virus therapy uses modified viruses that can infect and destroy cancer cells. Viruses are infectious agents that can invade living cells and hijack their genetic machinery, enabling the viruses to replicate within the cells. These viruses can be engineered to specifically target cancer cells while minimizing their ability to infect healthy, normal cells. Once they infect the cancer cells, these oncolytic viruses cause the cells to rupture, releasing new viruses and cancer antigens. This not only destroys the tumor, but also activates the immune system to eliminate remaining cancer cells, both at the primary site and potentially in distant metastases. In 2015, the U.S. FDA approved the first oncolytic virus therapy. Talimogene laherparepvec (T-VEC), indicated for the treatment of melanoma, uses a genetically modified herpes simplex virus designed to specifically infect and kill cancer cells while stimulating an immune response against the tumor. By harnessing the patient’s own immune system to fight cancer, immunotherapy offers a new, and often more durable approach than conventional therapies like chemotherapy. It has shown remarkable success and long-lasting responses in numerous cancer types, including advanced and treatment-resistant cancers, marking a revolutionary shift in cancer care with improved survival rates and enhanced quality of life. From immune checkpoint inhibitors and monoclonal antibodies to CAR T-cells and cancer vaccines, each modality offers unique mechanisms of action. As our understanding of the immune system and the mechanisms through which cancer cells evade it continues to advance, immunotherapy is expected to become an integral part of standard cancer care, offering new hope to patients who previously had limited treatment options and improving outcomes for patients worldwide.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.