Cancer drug resistance is a major barrier to the successful treatment of various cancers. Although many cancers are initially responsive to anti-cancer drugs designed to kill or inhibit their growth, cancer cells can adapt and become less responsive, or even completely resistant, to these drugs over time. This resistance can arise through a variety of mechanisms, and understanding these mechanisms is key to developing more effective treatment strategies.
Mechanisms of drug resistance
-
Tumor heterogeneity: Cancer is a heterogenous disease. Tumors are made up of a diverse population of cancer cells with varying genetic profiles and mutations. This variability means that a single therapy may not be effective against all cancer cells, as some may respond while others may be resistant. As a result of selective pressure on resistant clones induced by therapy, surviving cells that are therapy resistant can re-populate the tumor, causing a relapse.
-
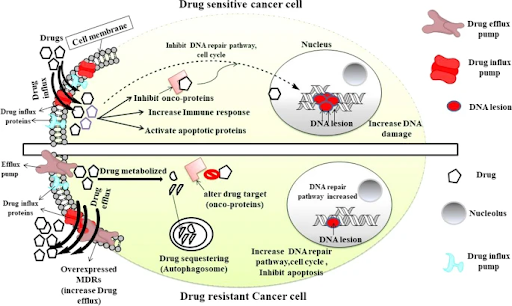
Drug efflux pumps: ATP-dependent drug efflux pumps, like P-glycoprotein, also play a key role in drug resistance by actively transporting drugs out of the cells. This process reduces the drug concentration inside the cells, decreasing its effectiveness. Cancer cells can increase the expression of these drug efflux pumps to expel drugs like doxorubicin and paclitaxel, lowering its intracellular concentration and hindering its ability to kill the cancer cells, leading to treatment failure.
-
Genetic mutations: As cancer cells divide and proliferate, they can acquire mutations that prevent the drug from binding effectively. For example, non-small cell lung cancer patients with EGFR exon 19 deletions or L858R point mutations may initially respond to EGFR tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib. Unfortunately, most patients treated with these first-generation EGFR TKIs will inevitably develop resistance, most commonly through EGFR T790M mutation, resulting in disease progression. To overcome this, a newer generation of EGFR TKIs like osimertinib, rociletinib and olmutinib have been designed to target tumors with EGFR T790M mutation.
-
Activation of pro-survival pathways: Cancer cells can also activate alternative pro-survival signaling pathways to bypass signaling pathways inhibited by the drug, allowing cancer cells to continue growing even in the presence of the drug. This phenomenon, known as pathway rewiring, can be seen in cancers such as melanoma, whereby patients with BRAF V600 mutations initially respond well to BRAF inhibitors such as vemurafenib. However, resistance can then develop through various mechanisms such as activation of the MAPK pathway via KRAS or NRAS secondary mutations, or increased pro-survival PI3K-AKT signaling, to promote continued survival and growth.
-
Enhanced DNA repair: Many therapies exert their effects by triggering cell death. Cancer cells can develop resistance by enhancing their DNA repair capabilities through activation of repair pathways such as homologous recombination repair and base excision repair, allowing them to fix the damage induced by DNA-damaging agents like platinum-based cisplatin. Key DNA repair proteins such as BRCA1, BRCA2 and PARP, are often overexpressed in resistant cancer cells, reducing the effectiveness of therapy.
-
Drug inactivation: Many anti-cancer drugs require metabolic activation to achieve clinical efficacy. However, cancer cells can develop resistance towards such treatments through decreased drug activation. An example of this can be seen in the treatment of acute myelogenous leukemia with cytarabine (AraC), a nucleoside drug that undergoes several phosphorylation steps to become activated AraC-triphosphate. Any down-regulation or mutation in this pathway can reduce AraC activation, reducing its efficacy.
-
Drug target alterations: Drugs are often designed to target a specific protein or receptor on cancer cells, but cancer cells can mutate these targets so that they can no longer bind to the drug or no longer be affected by it. For instance, HER2-positive breast cancer initially responds well to targeted therapy trastuzumab (Herceptin). But over time, some cancer cells may acquire changes in the HER2 receptor that prevent the drug from binding effectively, leading to resistance.
-
Epigenetic modifications: Changes in gene expression, without altering the DNA sequence, can also contribute to drug resistance. These changes can silence tumor suppressor genes, making cancer cells more resistant to drugs that normally trigger cell death. Additionally, DNA methylation and histone modifications can also regulate the expression of drug-metabolizing enzymes, which affects the metabolic process of anti-cancer drugs. For instance, DNA methylation suppresses the expression of UGT1A1, a metabolizing enzyme involved in the inactivation of irinotecan’s active metabolite, SN-38. Findings revealed that the demethylation at UGT1A1 promoter site is associated with tumor cells having significantly higher SN-38 glucuronidation capability and this could contribute to lower response of cancer cells to the drug.
-
Tumor microenvironment: Tumors are often surrounded by a complex environment of blood vessels, immune cells, fibroblasts, and other factors that can influence how cancer cells respond to therapy. For example, hypoxia (low oxygen levels) can limit effectiveness of certain chemotherapy drugs by impeding drug delivery or up-regulating anti-apoptotic proteins, making tumors less sensitive to drug-induced cell death. Moreover, tumor cells can induce the reprogramming of stromal and immune cells to secrete different factors such as cytokines to evade the immune system, suppress cell death and promote cell survival.
-
Cancer stem cells: These cells have stem-like properties, including the ability to self-renew and differentiate into various cell types, and are often more resistant to chemotherapy and radiation. A major factor in their drug resistance is their ability to remain in a quiescent or dormant state during treatment, enabling them to evade the cytotoxic effects of drugs that primarily target rapidly dividing cells. Due to their unique properties, cancer stem cells can survive treatments such as chemotherapy and radiation that kill the bulk of tumor cells, persist in the body, and contribute to tumor recurrence and relapse.
Strategies to overcome drug resistance
In summary, drug resistance is multifaceted and remains a major challenge in cancer treatment. Understanding the complex mechanisms of drug resistance is crucial in developing more effective and durable therapies. One promising approach to overcome drug resistance is combination therapy, which uses multiple drugs that target different mechanisms or pathways. By targeting multiple points of vulnerability within the cancer cells, combination therapies can reduce the chances of resistance development and enhance treatment outcomes. Precision medicine also plays an important role in mediating drug resistance. Genomic profiling using next-generation sequencing can help to identify specific mutations in cancers, allowing for personalized treatment strategies. This approach helps to reduce the likelihood of drug resistance by tailoring therapies to target specific mutations, improving treatment efficacy.
Canary Oncoceutics has a steadfast commitment to three fundamental pillars: advancing scientific knowledge, fostering collaboration, and ultimately, enhancing the lives of cancer patients worldwide. From cutting-edge research to impactful clinical advancements, Canary Oncoceutics aims to illuminate the transformative potential of tailored cancer treatments. Join us on this journey towards a future where every cancer patient receives personalized, effective treatment tailored to their unique needs.